Abstract
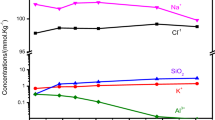
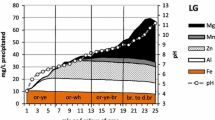
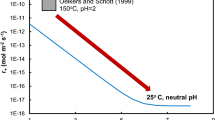
This paper explores how dissolution and precipitation reactions are coupled in batch reactor experimental systems at elevated temperatures. This is the fifth paper in our series of “Coupled Alkali Feldspar Dissolution and Secondary Mineral Precipitation in Batch Systems.” In the previous four papers we presented batch experiments of alkali-feldspar hydrolysis and explored the coupling of dissolution and precipitation reactions (Fu et al. in Chem Geol 91:955–964, 2009; Zhu and Lu in Geochim Cosmochim Acta 73:3171–3200, 2009; Zhu et al.in Geochim Cosmochim Acta 74:3963–3983, 2010; Lu et al. in Appl Geochem 30:75–90, 2013). Here, we present the results of additional K-rich feldspar hydrolysis experiments at 150 °C. Our solution chemistry measurements have constrained feldspar dissolution rates, and our high resolution transmission electron microscopy work has identified boehmite precipitation. Reaction path modeling of K-feldspar dissolution and boehmite precipitation simulated the coupled reactions, but only with forced changes of boehmite rate law in the middle of experimental duration. The results which are reported in this article lend further support to our hypothesis that slow secondary mineral precipitation explains part of the well-known apparent discrepancy between lab measured and field estimated feldspar dissolution rates (Zhu et al. in Water–rock interaction, 2004).













Similar content being viewed by others
References
Aagaard P, Helgeson HC (1982) Thermodynamic and kinetic constraints on reaction rates among minerals and aqueous solutions. I. Theoretical considerations. Am J Sci 282:237–285
Beig MS, Lüttge A (2006) Albite dissolution kinetics as a function of distance from equilibrium: implications for natural feldspar weathering. Geochim Cosmochim Acta 70:1402–1420
Bénézeth P, Palmer DA, Wesolowski DJ (2008) Dissolution/precipitation kinetics of boehmite and gibbsite: application of a pH-relaxation technique to study near-equilirbium rates. Geochim Cosmochim Acta 72:2429–2453
Blum A, Stillings L (1995) Feldspar dissolution kinetics. In: Brantley SL, White AR (eds) Chemical weathering rates of silicate minerals. Mineralogical Society of America, Washington, pp 291–346
Brantley SL (1992) Kinetics of dissolution and precipitation-experimental and field results. In: Kharaka Y, Maest A (eds) Proceedings of the seventh international conference on water-rock interactions, Park City, Utah. Balkema, Rotterdam, pp 465–469
Burch TE, Nagy KL, Lasaga AC (1993) Free energy dependence of albite dissolution kinetics at 80 °C and pH 8.8. Chem Geol 105:137–162
Carroll SA, Knauss KG (2005) Dependence of labradorite dissolution kinetics on CO2(aq), Al(aq), and temperature. Chem Geol 217:213–225
Cubilas P, Kohler S, Prieto M, Causserand C, Oelkers EH (2005) How do mineral coating affect dissolution rates? An experimental study of coupled CaCO3 dissolution–CaCO3 precipitation. Geochim Cosmochim Acta 69:5459–5476
Daval D, Sissmann O, Menguy N, Saldi GD, Guyot F, Martinez I, Corvisier J, Garcia B, Machouk I, Knauss KG, Hellmann R (2011) Influence of amorphous silica layer formation on the dissolution rate of olivine at 90° C and elevated pCO2. Chem Geol 284:193–209
Deer W, Howie R, Zussman J (1992) An introduction to the rock forming minerals, 2nd edn. Longman Scientific and Technical Group, Inc, Oceanside
Drever JI, Clow DW (1995) Weathering rates in catchments. In: White AF, Brantley SL (eds) Chemical weathering rates of silicate minerals. Mineralogical Society of America, New York, pp 463–481
Fu Q, Lu P, Konishi H, Dilmore R, Xu H, Seyfried WE Jr, Zhu C (2009) Coupled alkali-feldspar dissolution and secondary mineral precipitation in batch systems: 1. New experiment data at 200 oC and 300 bars. Chem Geol 91:955–964
Ganor J, Lu P, Zheng Z, Zhu C (2007) Bridging the gap between laboratory measurements and field estimations of weathering using simple calculations. Environ Geol 53:599–610
Gautier J-M, Oelkers EH, Schott J (1994) Experimental study of K-feldspar dissolution rates as a function of chemical affinity at 150 °C and pH 9. Geochim Cosmochim Acta 58:4549–4560
Gautier JM, Oelkers EH, Schott J (2001) Are quartz dissolution rates proportional to BET surface areas? Geochim Cosmochim Acta 65:1059–1070
Haar L, Gallagher JS, Kell GS (1984) NBS/NRC steam tables: thermodynamic and transport properties and computer programs for vapor and liquid states of water in SI units. Hemisphere Publishing Corporation, New York 320p
Harouiya N, Oelkers EH (2004) An experimental study of the effect of aqueous fluoride on quartz and alkali-feldspar dissolution rates. Chem Geol 205:155–167
Heinemann S, Wirth R, Dresen G (2003) TEM study of a special grain boundary in a synthetic K-feldspar bicrystal: manebach Twin. Phys Chem Miner 30:125–130
Hellmann R, Penisson JM, Hervig RL, Thomassin JH, Abrioux MF (2003) An EFTEM/HRTEM high-resolution study of the near surface of labradorite feldspar altered at acid pH: evidence for interfacial dissolution-reprecipitation. Phys Chem Miner 30:192–197
Hellmann R, Tisserand D (2006) Dissolution kinetics as a function of the Gibbs free energy of reaction: an experimental study based on albite feldspar. Geochim Cosmochim Acta 70:364–383
Hemingway BS, Robie RA, Apps JA (1991) Revised values for the thermodynamic properties of boehmite, AlO(OH), and related species and phases in the system Al-H-O. Am Mineral 76:445–457
Hereford AG, Keating E, Guthrie G, Zhu C (2007) Reactions and reaction rates in the aquifer beneath Pajarito Plateau, north-central New Mexico. Environ Geol 52:965–977
Ho PC, Bianchi H, Palmer DA, Wood RH (2000) Conductivity of dilute aqueous electrolyte solutions at high temperatures and pressures using a flow cell. J Solut Chem 29:217–235
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343
Koroleff F (1976) Determination of silicon. In: Grasshoff K (ed) Methods of seawater analysis. Spring Verlag, Newyork, pp 149–158
Lasaga AC (1981a) Rate laws of chemical reactions. In: Lasaga AC, Kirkpatrick RJ (eds) Kinetics of geochemical processes. Mineralogical Society of America, Washington, pp 1–68
Lasaga AC (1981b) Transition state theory. In: Lasaga AC, Kirkpatrick RJ (eds) Kinetics of geochemical processes. Mineralogical Society of America, Washington, pp 135–169
Lasaga AC (1998) Kinetic theory in the earth sciences. Princeton University Press, New York
Li L, Steefel CI, Yang L (2008) Scale dependence of mineral dissolution rates within single pores and fractures. Geochim Cosmochim Acta 72:360–377
Lu P, Fu Q, Seyfried WE Jr, Hedges SW, Soong Y, Jones K, Zhu C (2013) Coupled alkali feldspar dissolution and secondary mineral precipitation in batch systems: 2. New experiments with supercritical CO2 and implications for carbon sequestration. Appl Geochem 30:75–90
McCollom TM, Shock EL (1997) Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim Cosmochim Acta 61:4375–4391
Murakami T, Kogure T, Kadohara H, Ohnuki T (1998) Formation of secondary minerals and its effects on anorthite dissolution. Am Mineral 83:1209–1219
Nagy KL (1995) Dissolution and precipitation kinetics of sheet silicates. In: White AF, Brantley SL (eds) Chemical weathering rates of silicate minerals. Mineralogical Society of America, Washington, pp 173–225
Nugent MA, Brantley SL, Pantano CG, Maurice PA (1998) The influence of natural mineral coatings on feldspar weathering. Nature 395:588–591
Oelkers EH (2001) General kinetic description of multioxide silicate mineral and glass dissolution. Geochim Cosmochim Acta 65:3703–3719
Oelkers EH, Schott J, Devidal JL (1994) The effect of aluminum, pH, and chemical affinity on the rates of aluminosilicate dissolution reactions. Geochim Cosmochim Acta 58:2011–2024
Paces T (1973) Steady-state kinetics and equilibrium between ground water and granitic rocks. Geochim Cosmochim Acta 37:2641–2663
Siegel DI, Pfannkuch HO (1984) Silicate dissolution influence on Filson Creek chemistry, northeastern Minnesota. Geol Soc Am Bull 95:1446–1453
Sverjensky DA, Shock EL, Helgeson HC (1997) Prediction of the thermodynamic properties of aqueous metal complexes to 5 Kb and 1000 °C. Geochim Cosmochim Acta 61:1359–1412
Tagirov B, Schott J (2001) Aluminum speciation in crustal fluids revisited. Geochim Cosmochim Acta 65:3965–3992
Velbel MA (1990) Influence of temperature and mineral surface characteristics on feldspar weathering rates in natural and artificial systems: a first approximation. Water Resour Res 26:3049–3053
White AF, Brantley SL (2003) The effect of time on the weathering of silicate minerals: why do weathering rates in the laboratory and field? Chem Geol 202:479–506
Zhu C (2005) In situ feldspar dissolution rates in an aquifer. Geochim Cosmochim Acta 69:1435–1453
Zhu C (2009) Geochemical modeling of reaction paths and geochemical reaction networks. In: Oelkers EH, Schott J (eds) Thermodynamics and kinetics of water-rock interaction. Mineralogical Society of America, Washington, pp 533–569
Zhu C, Blum AE, Veblen DR (2004) Feldspar dissolution rates and clay precipitation in the Navajo aquifer at Black Mesa, Arizona, USA. In: Wanty RB, Seal RRI (eds) Water-rock interaction. August Aimé Balkema, Saratoga Springs, pp 895–899
Zhu C, Lu P (2009) Alkali feldspar dissolution and secondary mineral precipitation in batch systems: 3. Saturation states of product minerals and reaction paths. Geochim Cosmochim Acta 73:3171–3200
Zhu C, Lu P, Zheng Z, Ganor J (2010) Coupled alkali feldspar dissolution and secondary mineral precipitation in batch systems: 4. Numerical modeling of kinetic reaction paths. Geochim Cosmochim Acta 74:3963–3983
Zhu C, Veblen DR, Blum AE, Chipera SJ (2006) Naturally weathered feldspar surfaces in the Navajo Sandstone aquifer, Black Mesa, Arizona: electron microscopic characterization. Geochim Cosmochim Acta 70:4600–4616
Acknowledgments
A research grant from the State Key Laboratory of Ore Deposits at the Institute of Geochemistry, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, P., Konishi, H., Oelkers, E. et al. Coupled alkali feldspar dissolution and secondary mineral precipitation in batch systems: 5. Results of K-feldspar hydrolysis experiments. Chin. J. Geochem. 34, 1–12 (2015). https://doi.org/10.1007/s11631-014-0029-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-014-0029-z