Abstract
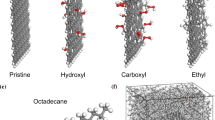
To understand the thermal conductivity improvement of the paraffin and graphite composite PCMs in micro-scale, the conformation characteristics of molecules with rising temperature was studied by molecular dynamics (MD) simulation. And then the structure and dynamics characteristics of the paraffin PCM, including the structural evolution, the self-diffusion coefficient, phase change properties and thermal conductivity, were analyzed. The results indicate that the distribution of the n-octadecane molecules is more regular in the region near the graphite layers, although the temperature is higher than the phase transition point, which means that the graphite layer has a significant absorption influence on the conformation of alkane molecules. Then, the self-diffusion coefficient of n-octadecane molecules increases with the increasing of temperature, which shows great agreement with the literature. Meanwhile, the self-diffusion coefficient of n-octadecane molecules staying far away from the graphite layers is larger than that of the molecules in the region near the graphite layer. In addition, the radial distribution function (RDF) was used to analyze the molecular interaction of the system at different temperatures. The sudden increase of R (ratio of the first peak value to the first valley value of the RDF) within the temperature range from 293 K to 313 K corresponds to the phase transition point, indicating that the solid-liquid phase transition occurs at the temperature range. Besides, the results indicate that the thermal conductivity of amorphous n-octadecane is about 2.5 times lower than that of the crystal n-octadecane with perfect structure. This investigation provides theoretical guidance for the study of the micro-mechanism of n-octadecane doped with graphite composite phase change materials.
Similar content being viewed by others
References
Farid M.M., Khudhair A.M., Sak R., Alhallaj S., A review on phase change energy storage: materials and applications. Energy Conversion & Management, 2004, 45(9): 1597–1615.
Fallahi A., Guldentops G., Tao M.J., Granados-Focil S., Van Dessel S., Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Applied Thermal Engineering, 2017, 127: 1427–1441.
Samer K., Michel J., Kheirabadi A., A comprehensive study of properties of paraffin phase change materials for solar thermal energy storage and thermal management applications. Energy, 2018, 162: 1169–1182.
Liu C., Rao Z., Zhao J., Huo Y., et al., Review on nano-encapsulated phase change materials: preparation, characterization and heat transfer enhancement. Nano Energy, 2015, 13: 814–826.
Fallahi A., Guldentops G., Tao M., et al., Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Applied Thermal Engineering, 2017, 127: 1427–1441.
An Z., Li J., Ding Y., et al., A review on lithium-ion power battery thermal management technologies and thermal safety. Journal of Thermal Science, 2017, 26(5): 391–412.
Liu C., Ma Z., Wang J., et al., Experimental research on flow and heat transfer characteristics of latent functional thermal fluid with microencapsulated phase change materials. International Journal of Heat and Mass Transfer, 2017, 115: 737–742.
Li C., Zhao X, Zhang B., et al., Stearic acid/copper foam as composite phase change materials for thermal energy storage. Journal of Thermal Science, 2020, 29: 492–502.
Hong Y., Ding S., Wu W., et al., Enhancing heat capacity of colloidal suspenssion using nanoscale encapsulated phase-change materials for heat transfer. ACS Applied Materials Interfaces, 2010, 2(6): 1685–1691.
Zhou X., Preparation and characterization of PEG/MDI/PVA copolymer as solid-solid phase change heat storage material. Journal of Applied Polymer Science, 2010, 113(3): 2041–2045.
Liu C., Zhang X., Lv P., et al., Experimental study on the phase change and thermal properties of paraffin/carbon materials based thermal energy storage materials. Phase Transitions, 2017, 90(7): 717–731.
Liang T., Zhang P., Yuan P., et al., A molecular dynamics study on the thermal conductivities of single- and multi-layer two-dimensional borophene. Nano Futures, 2019, 3: 015001.
Lafdi K., Mesalhy O., Elyafy A., Graphite foams infiltrated with phase change materials as alternative materials for space and terrestrial thermal energy storage applications. Carbon, 2008, 46(1): 159–168.
Sarı A., Karaipekli A., Thermal conductivity and latent heat thermal energy storage characteristics of paraffin/expanded graphite composite as phase change material. Applied Thermal Engineering, 2007, 27(8–9): 1271–1277.
Yuan P., Zhang P., Liang T., et al., Effects of functionalization on energy storage properties and thermal conductivity of graphene/n-octadecane composite phase change materials. Journal of Materials Science, 2019, 54(2): 1488–1501.
Yuan P., Zhang P., Liang T., et al., Effects of surface functionalization on thermal and mechanical properties of graphene/polyethylene glycol composite phase change materials. Applied Surface Science, 2019, 485: 402–412.
Zhong Y., Guo Q., Li S., et al., Heat transfer enhancement of paraffin wax using graphite foam for thermal energy storage. Solar Energy Materials and Solar Cells, 2010, 94(6): 1011–1014.
Li S., Song Y., Song Y., et al., Carbon foams with high compressive strength derived from mixtures of mesocarbon microbeads and mesophase pitch. Carbon, 2007, 45(10): 2092–2097.
Xiang J., Drzal L., Investigation of exfoliated graphite nanoplatelets (xGnP) in improving thermal conductivity of paraffin wax-based phase change material. Solar Energy Materials & Solar Cells, 2015, 95(7): 1811–1818.
Fei C., Wen R., Huang Z., et al., Preparation and analysis of lightweight wall material with expanded graphite (EG)/paraffin composites for solar energy storage. Applied Thermal Engineering, 2017, 120: 107–114.
Wu W., Wu W., Wang S., Thermal management optimization of a prismatic battery with shape-stabilized phase change material. International Journal of Heat and Mass Transfer, 2018, 121: 967–977.
Jiang G., Huang J., Preparation thermal management of expanded graphite/paraffin composite for Li-ion battery. Journal of Materials Engineering, 2017, 45(7): 41–47.
Luo J., Yin H., Li W., et al., Numerical and experimental study on the heat transfer properties of the composite paraffin/expanded graphite phase change material. International Journal of Heat & Mass Transfer, 2015, 84: 237–244.
Liu X., Lin C., Rao Z., Diffusion and thermal conductivity of the mixture of paraffin and polystyrene for thermal energy storage: A molecular dynamics study. Journal of the Energy Institute, 2017, 90(4): 534–543.
Liu X., Rao Z., Thermal diffusion and phase transition of n-octadecane as thermal energy storage material on nanoscale copper surface: A molecular dynamics study. Journal of the Energy Institute, 2019, 92(1): 161–176.
Kousksou T., Jamil A., Rhafiki T., et al., Paraffin wax mixtures as phase change materials. Solar Energy Materials & Solar Cells, 2010, 94: 2158–2165.
Marbeuf A., Brown R., Molecular dynamics in n-alkanes: premelting phenomena and rotator phases. Journal of Chemical Physics, 2006, 124: 054901.
Wentzela N., Milner S., Crystal and rotator phases of n-alkanes: a molecular dynamics study. Journal of Chemical Physics, 2010, 132: 044901.
Shimizu T., Yamamoto T., Melting and crystallization in thin film of n-alkanes: a molecular dynamics simulation. Journal of Chemical Physics, 2000, 113: 3351–3359.
Mavrantza I., Prentzas D., Mavrantzas V., et al., Detailed atomistic molecular-dynamics simulation of the orthorhombic phase of crystalline polyethylene and alkane crystals. Journal of Chemical Physics, 2001, 115: 3937–3950.
Esselink K., Hilbers P., Beest B., Molecular dynamics study of nucleation and melting of n-alkanes. Journal of Chemical Physics, 1994, 101: 9033–9041.
Babaei H., Keblinski P., Khodadadi J., Thermal conductivity enhancement of paraffins by increasing the alignment of molecules through adding CNT/graphene. International Journal of Heat and Mass Transfer, 2013, 58(1–2): 209–216.
Rao Z., Wang S., Peng F., Molecular dynamics simulations of nano-encapsulated and nanoparticle-enhanced thermal energy storage phase change materials. International Journal of Heat & Mass Transfer, 2013, 66: 575–584.
Feng H., Gao W., Nie J., MD simulation of self-diffusion and structure in somen-alkanes over a wide temperature range at high pressures. Journal of Molecular Modeling, 2013, 19(1): 73–82.
Sun H., COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. The Journal of Physical Chemistry B, 1998, 102: 7338–7364.
Zhang Y., Feller S., Brooks B., et al., Computer simulation of liquid/liquid interfaces. I. Theory and application to octane/water. The Journal of Chemical Physics, 1995, 103(23): 10252–10266.
Hellmann R., Bich E., Vogel E., et al., Calculation of the transport and relaxation properties of methane. I. Shear viscosity, viscomagnetic effects, and self-diffusion. The Journal of Chemical Physics, 2008, 129(6): 064302.
Hofmann D., Fritz L., Ulbrich J., et al., Detailed-atomistic molecular modeling of small molecule diffusion and solution processes in polymeric membrane materials. Macromolecular Theory & Simulations, 2000, 9(6): 293–327.
Fu Y., Liu Y., Zhang L., et al., Molecular simulation of the different configuration of polypropylene. Journal of Molecular Science, 2009, 25(1): 1–1.
Müller-Plathe F., A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. Journal of Chemical Physics, 1997, 106(14): 6082–6085.
Nieto-Draghi C., Avalos J., Non-equilibrium momentum exchange algorithm for molecular dynamics simulation of heat flow in multicomponent systems. Molecular Physics, 2003, 101(14): 2303–2307.
Jund P., Jullien R., Molecular-dynamics calculation of the thermal conductivity of vitreous silica. Physical Review B, 2007, 59(21): 13707–13711.
Müller T., Müller-Plathe F., Heat transport through a biological membrane—an asymmetric property? Technical issues of nonequilibrium molecular dynamics methods. International Journal of Quantum Chemistry, 2010, 111(7/8): 1403–1418.
Peng D., Robinson D., A new two-constant equation of state. Industrial & Engineering Chemistry Research, 1976, 15(1): 59–64.
Abraham F., An isothermal isobaric computer simulation of the supercooled liquid-glass transition region is the short range order. The Journal of Chemical Physics, 1980, 72(2): 359–365.
Kim T., Kelton K., Structural study of supercooled liquid transition metals. The Journal of Chemical Physics, 2007, 126(5): 054513.
Jakse N., Pasturel A., Dynamic aspects of the liquid-liquid phase transformation in silicon. The Journal of Chemical Physics, 2008, 129(10): 104503.
An M., Demir B., Wan X., et al., Predictions of thermo-mechanical properties of cross-linked polyacrylamide hydrogels using molecular simulations. Advanced Theory and Simulations, 2019, 2(3): 1800153.
Demir B., In silico study of bio-based epoxy precursors for sustainable and renewable thermosets. Polymer, 2020, 191: 122253.
Qiu F., Ren L., Wang J., Prediction of glass transition temperature of chitosan through molecular dynamics simulation. CIESC Journal, 2012, 63(7): 2285–2289.
Lechner W., Dellago C., Accurate determination of crystal structures based on averaged local bond order parameters. The Journal of Chemical Physics, 2008, 129(11): 114707.
Dhakal S., Sureshkumar R., Anomalous diffusion and stress relaxation in surfactant micelles. Physicla Review E, 2017, 96: 012605.
Zhang X., Jiang J., Thermal conductivity of zeolitic imidazolate framework-8: a molecular simulation study. Journal of Physical Chemistry C, 2013, 117(36): 18441–18447.
Lin C., Rao Z., Thermal conductivity enhancement of paraffin by adding boron nitride nanostructures: A molecular dynamics study. Applied Thermal Engineering, 2017, 110: 1411–1419.
Kelkar M., Rafferty J., Maginn E., et al., Prediction of viscosities and vapor-liquid equilibria for five polyhydric alcohols by molecular simulation. Fluid Phase Equilibria, 2007, 260(2): 218–231.
Fan L., Khodadadi J., Thermal conductivity enhancement of phase change materials for thermal energy storage: A review. Renewable and Sustainable Energy Reviews, 2011, 15(1): 24–46.
Acknowledgements
This study is financially supported by National Natural Science Foundation of China (No. 51676037).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, T., Xu, B. & Chen, Z. Effect of Graphite Layers on the Conformation and Thermal Conductivity of n-octadecane: A Molecular Dynamics Study. J. Therm. Sci. 30, 1789–1802 (2021). https://doi.org/10.1007/s11630-021-1506-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11630-021-1506-4