Abstract
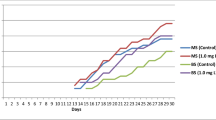
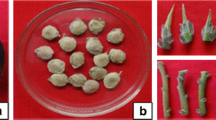
Aflatunia ulmifolia (Franch.) Vassilcz. (Rosaceae) is an ornamental plant commonly used for landscaping and is known to form hybrids with other species that have characteristics like frost-resistant and non-rotting rootstocks. However, it is listed as an endangered species in Kazakhstan and is difficult to grow through traditional propagation; thus, in the present study, in vitro regeneration using seed and nodal explants was attempted. Murashige and Skoog (MS), Woody Plant Medium, and Gamborg medium supplemented with different plant growth regulators were utilized. Maximum germination (51.7±4.4%) was achieved by supplementing MS medium with 1.0 mg L-1 6-benzyladenine (BA) in which maximum shoot length (1.91±0.11 cm), leaves per shoot (8.48±0.32), and shoots per seed (1.83±0.15) were also observed after 12 wk. Comparatively, nodal explants proved better as 58.9±2.9% bud break was noted, inducing 2.84±0.18 shoots per explant with 0.74±0.01 cm length and 5.96±0.17 leaves per shoot at 1.0 mg L-1 BA after 8 wk. Nodal derived shoots were further multiplied on MS medium in the presence of 1.5 mg L-1 BA where 3.90±0.32 shoots (96.7% response) formed with 1.33±0.04 cm length after 4 wk. These shoots were rooted in MS media augmented with 0.5 mg L-1 indole-3-acetic acid, which induced 3.5±0.3 roots per shoot (83.3% response) with 3.8±0.1 cm root length at the end of 4 wk. The plantlets were then transferred to peat:vermiculite (1:1 w/w) for hardening and acclimatization and were finally transferred in the open ground with 69.4% survival rate after 1 mo. This micropropagation protocol will be helpful for the conservation of the genetic resources and biodiversity for A. ulmifolia.


Similar content being viewed by others
Data availability
All data related to experimental work are included in this article.
References
Abdulina SA (1999) List of vascular plants of Kazakhstan [Spisok sosudistykh rasteniy Kazakhstana]. Steka, Almaty, p 187
Ahmad Z, Yadav V, Shahzad A, Emamverdian A, Ramakrishnan M, Ding Y (2022) Micropropagation, encapsulation, physiological, and genetic homogeneity assessment in Casuarina equisetifolia. Front Plant Sci 13:905444
Ahmed MEAE (2022) In vitro propagation and improving accumulation of coumarin in Lycium barbarum, a rare plant in the flora of Egypt. Bull Natl Res Cent 46:220
Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411:1053–1057
Arab MM, Yadollahi A, Shojaeiyan A, Shokri S, Ghojah SM (2014) Effects of nutrient media, different cytokinin types and their concentrations on in vitro multiplication of G × N15 (hybrid of almond × peach) vegetative rootstock. J Genet Eng Biotechnol 12:81–87
Asmar SA, Castro EM, Pasqual M, Pereira FJ, Soares JDR (2013) Changes in leaf anatomy and photosynthesis of micropropagated banana plantlets under different silicon sources. Sci Hortic 161:328–332
Avdeev VI (2014) Protein markers of Louiseania ulmifolia (Franch.) Pachom. plant and its intergeneric hybrids. Izvestia Orenburg State Agrarian Univ 1:8–11
Balapour Z, Moghaddam HH, Zarei M, Mollashahi M (2020) Micro propagation of penta rootstock (Prunus domestica) in the two culture media (MS and B5). Plant Prod 42:441–454
Bhosale UP, Dubhashi SV, Mali NS, Rathod HP (2011) In vitro shoot multiplication in different species of banana. Asian J Plant Sci Res 1:23–27
Bhowmik TK, Rahman MM (2017) Effect of different basal media and PGRs on in vitro seed germination and seedling development of medicinally important orchid Cymbidium aloifolium (L.) Sw. J Pharmacogn Phytochem 6:167–172
Borkheyli MM, Miri SM, Nabigoland A (2021) In vitro multiplication and rooting of GF677 rootstock. J Hortic Postharvest Res 4:243–252
Bramhanapalli M, Thogatabalija L, Gudipalli P (2017) Efficient in vitro plant regeneration from seedling-derived explants and genetic stability analysis of regenerated plants of Simarouba glauca DC. by RAPD and ISSR markers. In Vitro Cell Dev Biol - Plant 53:50–63
Chiruvella KK, Mohammed A, Ghanta RG (2014) Factors influencing the seed germination of Soymida febrifuga (Roxb.) A. Juss. (Meliaceae). Trakia J Sci 2:121–131
Chokheli VA, Bakulin SD, Ermolaeva OY, Kozlovsky BL, Dmitriev PA, Stepanenko VV, Kornienko IV, Bushkova AA, Rajput VD, Varduny TV (2023) Investigation of growth factors and mathematical modeling of nutrient media for the shoots multiplication in vitro of rare plants of the Rostov region. Hortic 9:60
Cui Y, Deng Y, Zheng K, Hu X, Zhu M, Deng X, Xi R (2019) An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. and Chalermglin) and assessment of genetic uniformity through DNA markers. Sci Rep 9:9634
Dangi B, Khurana-Kaul V, Kothari SL, Kachhwaha S (2014) Micropropagtion of Terminalia bellerica from nodal explants of mature tree and assessment of genetic fidelity using ISSR and RAPD markers. Physiol Mol Biol Plants 20:509–516
Darwesh MA, Naser AA, Habba EE, Taha LH, Ahmed MMG, El-Assaly RMB (2017) In vitro propagation protocol for improving African mahogany (Khaya senegalensis) endangered tree. J Biol Sci 17:235–246
de Souza Ferrari MP, da Cruz RMS, dos Santos QM, de Andrade MM, Alberton O, Magalhaes HM (2020) Efficient ex vitro rooting, acclimatization, and cultivation of Curcuma longa L. from mycorrhizal fungi. J Crop Sci Biotechnol 23:469–482
Ebrahimzadeh A, Fathollahzadeh M, Aazami MA, Hassanpouraghdam MB (2022) In vitro direct and indirect regeneration of plants from nodal and petiole explants in Pelargonium odoratissimum (L.) Herit. Acta Agric Slov 118:1–7
Eremin GV, Eremin VG (2015) Use of the genetic diversity of wild Prunus L. species in breeding of clonal rootstocks of stone fruit crops. Proc Appl Bot Genet Breed 176:416–428
Gallo CM, Radmann EB, Ritterbusch CW, da Rosa FÂ, Bianchi VJ, Peters JA (2017) The effect of the culture media components by in vitro multiplication ‘Mr. S 2/5’ rootstock. Rev Cienc Agric 15:9–16
Gamborg OL, Eveleigh DE (1968) Culture methods and detection of glucanases in suspension cultures of wheat and barley. Can J Biochem 46:417–421
Garapov DS, Puchkin IA (2014) Frost-hardiness and asphyxiation resistance of oriental plum (Prunus ussuriensis) on interstems in the Altai forest-steppe zone. Vestnik of Lobachevsky University of Nizhni Novgorod 4:210–213
Gorbunov AB, Simagin VS, Fotev YV, Boyarskikh IG, Snakina TI, Lokteva AV, Asbaganov SV, Belousova VP (2013) Introduction of non-traditional fruit, berry and vegetable plants in Western Siberia. Geo Publ, Novosibirsk, p 290
Grzegorczyk-Karolak I, Kuźma Ł, Wysokińska H (2015) The effect of cytokinins on shoot proliferation, secondary metabolite production and antioxidant potential in shoot cultures of Scutellaria alpina. Plant Cell Tiss Org Cult 122:699–708
Hussain A, Ahmed I, Nazir H, Ullah I (2012) Plant tissue culture: current status and opportunities. In: Leva A, Rinaldi LMR (eds) Recent advances in plant in vitro culture. InTech, Rijeka, pp 1–28
Iwuagwu MO, Nwosu NN (2018) Performance of in vitro cassava (Manihot esculenta Crantz) plantlets weaned with locally sourced substrates. Int J Environ Agric Biotechnol 3:663–669
Kim TD, Kim NH, Park EJ, Lee NN (2021) High-frequency regeneration of plants in vitro from seedling-derived apical bud explants of Tilia mandshurica Rupr. & Maxim. J Plant Biotechnol 48:54–61
Kirillov V, Ivashchenko A, Stikhareva T, Serafimovich M, Daulenova M, Bystriakova N (2023b) Changes in species diversity and floristic composition over sixty years in plant communities with Aflatunia ulmifolia in mountainous Kazakhstan. Phytocoenologia 51:313–329
Kirillov V, Pathak A, Patel SR, Daulenova M, Dyussembekova D, Stikhareva T, Rakhimzhanov A, Kakimzhanova A (2023a) Micropropagation of Cotoneaster melanocarpus Fisch. ex A.Blytt: an economically important ornamental plant. In Vitro Cell Dev Biol – Plant 59:147–153
Kirillov V, Pathak A, Stikhareva T, Ercisli S, Daulenova M, Kazangapova N, Rakhimzhanov A (2022a) In vitro propagation and ex vitro rooting of Euonymus verrucosus Scop. (Celastraceae) – a rare species of Kazakhstan flora on the southern border of its areal. J For Res 27:289–296
Kirillov V, Pathak A, Zholdasbayev M, Atazhanova G, Sapiyeva A, Stikhareva T, Serafimovich M, Daulenova M (2022b) HPLC and GC/MS analysis of Prunus ulmifolia Franch. (syn. Aflatunia ulmifolia (Franch.) Vassilcz.) leaves growing in South-Eastern Kazakhstan. Nat Prod Res. 1-9 https://doi.org/10.1080/14786419.2022.2137801
Kirillov V, Stikhareva T, Ivashchenko A, Sitpayeva G, Kuliyev A, Serafimovich M, Daulenova M, Zholdasbayev M (2022c) Expanding the knowledge about Aflatunia ulmifolia (Franch.) Vassilcz. (Rosaceae), a rare forest species of Central Asia. Bot Lett 169:71–82
Kose MSH, Dogan M, Sadi G (2021) Enhanced in vitro shoot proliferation through nodal explants of Staurogyne repens (Nees) Kuntze. Biol 76:1053–1061
Li H, Zhang D (2018) In vitro seed germination of Kalmia latifolia L. hybrids: a means for improving germination and speeding up breeding cycle. HortScience 53:535–540
Lim MY, Lee EJ, Jana S, Sivanesan I, Jeong BR (2012) Effect of potassium silicate on growth and leaf epidermal characteristics of begonia and pansy grown in vitro. Kor J Hort Sci Technol 30:579–585
Mahender A, Mahesh DM, Murthy EN (2014) In vitro seed germination and development of Butea monosperma (Lam.) Taub. Var. lutea (Willt.): a step for rehabilitation. Int J Multidisciplin Curr Res 2:297–301
Matt A, Jehle JA (2005) In vitro plant regeneration from leaves and internode sections of sweet cherry cultivars (Prunus avium L.). Plant Cell Rep 24:468–476
Matyunin MN (2017) Promising clonal stocks for stone crops in Siberia. Works of the State Nikit Botan Gard 144:81–84
Mezhenskiy VN (2008) The use of hybrids Louiseania ulmifolia (Franch.) Pachom. × Microcerasus tomentosa (Thunb.) Erem. et Yushev in improving the assortment of M. tomentosa. Pomicult Small Fruit Cult Russ 20:163–166
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nigmatyanova SE, Mursalimova GR (2018) The influence of grafting time of Rosaceae L. species on the rooting of softwood cuttings. Contemporary Hortic 1:55–61 (Russian)
Online document: IPNI, International Plant Names Index (2023) The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Herbarium. http://www.ipni.org. Cited 15 March 2023
Otroshy M, Zamani A, Khodambashi M, Ebrahimi M, Struik PC (2009) Effect of exogenous hormones and chilling on dormancy breaking of seeds of asafoetida (Ferula asafoetida L.). Res J Seed Sci 2:9–15
Pathak AR, Joshi AG, Shrivastava N, Sharma P (2017) Regeneration and chemical profiling in Hemidesmus indicus (L.) R. Br. S Afr J Bot 113:413–420
Pavlov NV (1961) Flora of Kazakhstan [Flora Kazakhstana]. Academy of Sciences of the Kazakh SSR Publ [Izdatelstvo Akademii nauk Kazakhskoy SSR], Alma-Ata, vol 4, p 548 (Russian)
Pavlov VN (1980) Vegetative cover of the Western Tien Shan [Rastitel'nyy pokrov Zapadnogo Tyan'-Shanya]. Moscow State University, Moscow, p 246
Polivanova OB, Bedarev VA (2022) Hyperhydricity in plant tissue culture. Plants 11:3313
Ramírez-Mosqueda MA, Cárcamo-Corona RG, Aguilar-Jiménez D, Bello-Bello JJ (2022) Micropropagation of agave (Agave potatorum Zucc.) through direct organogenesis. Agrociencia. https://doi.org/10.47163/agrociencia.v56i6.2823
Rathore JS, Rathore V, Shekhawat NS, Singh RP, Liler G, Phulwaria M, Dagla HR (2004) Micropropagation of woody plants. In: Srivastava PS, Srivastava S (eds) Plant biotechnology and molecular markers. Anamaya Publishers, New Delhi, pp 195–204
Rathwell R, Shukla MR, Jones AMP, Saxena PK (2016) In vitro propagation of cherry birch (Betula lenta L.). Can J Plant Sci 96:571–578
Ružić DV, Vujović TI (2008) The effects of cytokinin types and their concentration on in vitro multiplication of sweet cherry cv. Lapins (Prunus avium L.). HortScience 35:12–21
Sadhu S, Jogam P, Thampu RK, Abbagani S, Penna S, Peddaboina V (2020) High efficiency plant regeneration and genetic fidelity of regenerants by SCoT and ISSR markers in chickpea (Cicer arietinum L.). Plant Cell Tiss Org Cult 141:465–477
Salem J, Hassanein A, El-Wakil DA, Loutfy N (2022) Interaction between growth regulators controls in vitro shoot multiplication in Paulownia and selection of NaCl-tolerant variants. Plants 11:498
Shekhawat MS, Kannan N, Manokari M, Ravindran CP (2015) In vitro regeneration of shoots and ex vitro rooting of an important medicinal plant Passiflora foetida L. through nodal segment cultures. J Genet Eng Biotechnol 13:209–214
Sivanesan I, Song JY, Hwang SJ, Jeong BR (2011) Micropropagation of Cotoneaster wilsonii Nakai - a rare endemic ornamental plant. Plant Cell Tiss Org Cult 105:55–63
Smith MAL, McCown BH (1983) A comparison of source tissue for protoplast isolation from three woody plant species. Plant Sci Lett 28:149–156
Sokolov SY, Svyazeva OA, Kubli VA (1980) Areas of distribution of trees and shrubs in the USSR [Arealy derev'ev i kustarnikov SSSR], vol 2. Polygonaceae - Rosaceae, Nauka Leningrad, p 144 (Russian)
Soundararajan P, Manivannan A, Cho YS, Jeong BR (2017) Exogenous supplementation of silicon improved the recovery of hyperhydric shoots in Dianthus caryophyllus L. by stabilizing the physiology and protein expression. Front. Plant Sci 8:738
The Red Data Book of Kazakhstan (2014) The 2nd edition revised and supplemented. Plants. Art Print XXI Publ, Astana, Kazakhstan, vol 2, p 452
Vodyanova SR, Tkachenko VI (1991) Winter-hardiness of trees, shrubs and spontaneous hybrids of local flora [Zimostoykost derevyev. kustarnikov i spontannykh gibridov mestnoy flory]. In: Tkachenko VI, Akhmatov KA (eds) Introduction of trees, shrubs and fruit plants to Kyrgyzstan [Introduktsiya derevyev, kustarnikov i plodovykh rasteniy v Kirgiziyu]. Bishkek, Ilim, pp 14–18
Zapryagaeva VI (1976) Forest resources of the Pamir-Alai [Lesnye resursy Pamiro-Alaya]. Science, Leningrad, p 594
Zenna FG (2020) An efficient protocol for in vitro propagation of purple-leaf plum (Prunus cerasifera). Egypt J Agric Res 98:435–451
Zulfiqar B, Nadeem AA, Touqeer A, Ishfaq AH (2009) Effect of explant sources and different concentrations of plant growth regulators on in in vitro shoot proliferation and rooting of avocado (Persea americana Mil) cv, Furete. Pak J Bot 41:2333–2346
Acknowledgements
This work was supported by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan under Grant No. AR09057922.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editor: Masaru Nakano.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kirillov, V., Pathak, A., Patel, S.R. et al. An efficient micropropagation protocol for an endangered tree species Aflatunia ulmifolia (Franch.) Vassilcz. In Vitro Cell.Dev.Biol.-Plant 60, 28–38 (2024). https://doi.org/10.1007/s11627-023-10392-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-023-10392-y