Abstract
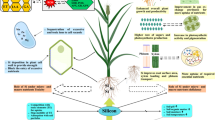
As two newly important components for plant tissue culture, the impacts of iron nanoparticle and potassium silicate were studied on the regeneration and growth of grape cuttings var. Khoshnaw under salinity condition. The treatments consisted of salinity stress (0, 50, and 100 mM NaCl), iron nanoparticles (0.0, 0.08, and 0.8 ppm) and potassium silicate (0, 1, and 2 mM) under an in vitro environment. The overall results indicated that salinity significantly (p ≤ 0.05) increased soluble carbohydrates and carotenoid contents. On one hand, it reduced all studied morphological and physiological traits including shoot number, shoot and root length, shoot and root fresh weight, root volume, and leaf area, along with relative water content (RWC) and chlorophylls’ content. On the other hand, the application of iron nanoparticles and potassium silicate, alone or in combination, could significantly compensate the deleterious effects of salinity on morphological traits, leading to increase their mean values compared to control condition (p ≤ 0.05). Soluble carbohydrate content showed negative significant (p ≤ 0.05) correlation with RWC, chlorophyll a, and all morphological parameters. Chlorophyll b and total chlorophyll contents showed positive significant (p ≤ 0.01) correlation with RWC. The application of higher concentrations of potassium silicate resulted in a greater ability of plants to tolerate salinity; moreover, the results suggest that moderate concentrations of iron nanoparticles may be more profitable for increasing salinity tolerance.



Similar content being viewed by others
References
Alexandre A, Meunier JD, Colin F, Koud JM (1997) Plant impact on the biogeochemical cycle of silicon and related weathering processes. Geochim Cosmochim Acta 61:677–682
Barhoumi Z, Rabhi M, Gharsalli M (2007) Effect of two nitrogen forms on the growth and iron nutrition of pea cultivated in presence of bicarbonate. J Plant Nutr 30:1953–1965
Daub ME (1986) Tissue culture and the selection of resistance to pathogens. Annu Rev Phytopathol 24:159–186
Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11:1–42
El-Agamy SZ, El-Mahdy TK, Mohamed AA (2008) In vitro propagation of some grape rootstocks. In: Proceedings of the first international symposium on biotechnology of fruit species (Biotechfruit 2008). Dresden, Germany, September 1–5 Abstract Book, pp 125–132
Elbotaty EMA (2012) Production of developed grape rootstocks using in vitro mutations. Dissertation, University of Cairo, Egypt
Fisarakis I, Chartzoulakis K, Stavrakas D (2001) Response of Sultana vines (V. vinifera L.) on six rootstocks to NaCl salinity exposure and recovery. Agric Water Manag 51:13–27
Flowers TJ, Yeo AR (1989) Effects of salinity on plant growth and crop yield. In: Cherry JH (ed) Environmental stress in plants. Springer, Berlin, pp 101–119
George EF, Hall MA, De Klerk GJ (2007) Plant propagation by tissue culture, vol 1. Springer, Berlin
Gong H, Zhu X, Chen K, Wang S, Zhang C (2005) Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci 169:313–321
Gupta R, Wall T, Baxter L (2007) Impact of mineral impurities in solid fuel combustion. Springer Science & Business Media, New York
Habibi G, Sarvary S (2015) The roles of selenium in protecting lemon balm against salt stress. Iran J Plant Physiol 5:1425–1433
Jaén J, de Saldana EG, Hernandez C (1999) Characterization of reaction products of iron and iron salts and aqueous plant extracts. Hyper Interact 122:139–145
Kotuby-Amacher J, Koenig R, Kitchen B (2000) Salinity and plant tolerance. Electronic Publication AG-SO-03, Utah State University Extension, Available via https://digitalcommons.usu.edu. Cited 4 Apr 2019
Liang Y, Chen Q, Liu Q, Zhang W, Ding R (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol 160:1157–1164
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker Sporns P (eds) Current protocols in food analytical chemistry (CPFA). Wiley, New York, pp F4.3.1 –F4.3.8,
Ma JF, Takahashi E (2002) Soil, fertilizer and plant silicon research in Japan. Elsevier, Amsterdam
Ma JF, Yamaji N, Mitani-Ueno N (2011) Transport of silicon from roots to panicles in plants. Proc Jpn Acad Ser B Phys Biol Sci 87:377–385
Malusá E, Sas-Paszt L, Ciesielska J (2012) Technologies for beneficial microorganisms inocula used as biofertilizers. Sci World J 2012:491206
Mozafari AA, Vafaee Y, Karami E (2015) In vitro propagation and conservation of Satureja avromanica Maroofi—an indigenous threatened medicinal plant of Iran. Physiol Mol Biol Plants 21:433–439
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nadi E, Aynehband A, Mojaddam M (2013) Effect of nano-iron chelate fertilizer on grain yield, protein percent and chlorophyll content of Faba bean (Vicia faba L.). Int J Biosci 3:267–272
Nwugo CC, Huerta AJ (2008) Silicon-induced cadmium resistance in rice (Oryza sativa). J Plant Nutr Soil Sci 17:841–848
Pati PK, Rath SP, Sharma M, Sood A, Ahuja PS (2006) In vitro propagation of rose—a review. Biotechnol Adv 24:94–114
Richmond KE, Sussman M (2003) Got silicon? The non-essential beneficial plant nutrient. Curr Opin Plant Biol 6:268–272
Rodrigues F, Duarte H, Domiciano G, Souza C, Korndörfer G, Zambolim L (2009) Foliar application of potassium silicate reduces the intensity of soybean rust. Australas Plant Pathol 38:366–372
Rolli E, Brunoni F, Marieschi M, Torelli A, Ricci A (2015) In vitro micropropagation of the aquatic fern Marsilea quadrifolia L. and genetic stability assessment by RAPD markers. Plant Biosys 149:7–14
Römheld V, Marschner H (1991) Function of micronutrients in plants. In: Mortdvedt JJ, Cox FR, Shuman LM, Welch RM (eds) Micronutrients in Agriculture. Soil Science Society of America, Madisonm USA pp 297–318
Sivanesan I, Jeong BR (2014) Silicon promotes adventitious shoot regeneration and enhances salinity tolerance of Ajuga multiflora Bunge by altering activity of antioxidant enzyme. Sci World J 2014:521703
Squashic SA, Hudy KM, Purdy DC (2012) Nutritional supplement for use under physiologically stressful conditions, Google Patents. https://patents.google.com/patent/US7901710B2/en. Accessed 2 Nov 2018
Tahir MA, Rahmatullah T, Aziz M, Ashraf S, Kanwal MM, Maqsood MA (2006) Beneficial effects of silicon in wheat (Triticum aestivum L.) under salinity stress. Pak J Bot 38:1715–1722
Taiz L, Zeiger E (2015) Plant physiology, 5th ed. Sinauer Associates, Sunderland, MA
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298–1301
Ursache-Oprisan M, Focanici E, Creanga D, Caltun O (2011) Sunflower chlorophyll levels after magnetic nanoparticle supply. Afr J Biotechnol 10:7092–7098
Vafaee Y, Ghaderi N, Khadivi A (2017) Morphological variation and marker-fruit trait associations in a collection of grape (Vitis vinifera L.). Sci Hortic 225:771–782
Van der Salm TP, Van der Toorn CJ, Hänisch ten Cate CH, Dubois LA, De Vries DP, Dons HJ (1994) Importance of the iron chelate formula for micropropagation of Rosa hybrida L‘Moneyway’. Plant Cell Tissue Organ Cult 37:73–77
Yaghubi K, Ghaderi N, Vafaee Y, Javadi T (2016) Potassium silicate alleviates deleterious effects of salinity on two strawberry cultivars grown under soilless pot culture. Sci Hortic 213:87–95
Yeo A, Yeo M, Flowers S, Flowers T (1990) Screening of rice (Oryza sativa L.) genotypes for physiological characters contributing to salinity resistance, and their relationship to overall performance. Theor Appl Genet 79:377–384
Zawadzka M, Orlikowska T (2006) The influence of FeEDDHA in red raspberry cultures during shoot multiplication and adventitious regeneration from leaf explants. Plant Cell Tissue Organ Cult 85:145–149
Zhu Z, Wei G, Li J, Qian Q, Yu J (2004) Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci 167:527–533
Funding
This work was supported by the University of Kurdistan under grant number [4.14364]
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Yong Eui Choi
Rights and permissions
About this article
Cite this article
Ghadakchi asl, A., Mozafari, A.a. & Ghaderi, N. Iron nanoparticles and potassium silicate interaction effect on salt-stressed grape cuttings under in vitro conditions: a morphophysiological and biochemical evaluation. In Vitro Cell.Dev.Biol.-Plant 55, 510–518 (2019). https://doi.org/10.1007/s11627-019-09988-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-019-09988-0