Abstract
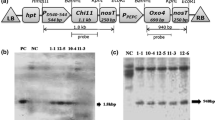
Agrobacterium-mediated transformation was used to introduce pathogenesis-related protein genes into scutellum-derived callus of rice (Oryza sativa L. subsp. indica ‘White Ponni’) as a means of increasing resistance to sheath blight disease caused by Rhizoctonia solani Kühn. Transformation with a tobacco osmotin (ap24) gene driven by the Cauliflower mosaic virus 35S promoter (P35S) yielded six single-copy transgenic lines. Homozygous T2 plants of all lines accumulated high levels of the ap24 transcript and the osmotin protein and reduced the percent disease index (PDI) of sheath blight disease from 100% down to 49–77%. Transformation with the binary plasmid pNSP2, which harbored the P35S-ap24 gene and a maize ubiquitin promoter (PUbi1)-driven rice chitinase (chi11) gene in the same T-DNA, yielded three single-copy transgenic lines: CO1, CO2, and CO3. Homozygous T2 plants of all three lines accumulated high levels of the chi11 transcript and the chitinase protein. Accumulation of ap24 transcript and osmotin protein was high in homozygous CO1 and CO2, but very low in CO3. At 7 d post infection with R. solani, the transgenic rice lines C18a (with chi11 alone), O8 (with ap24 alone), and CO1, CO2, and CO3 (with chi11 + ap24) reduced the sheath blight PDI from 100% to 64.2, 57.2, 43.3, 50.2, and 58.6%, respectively. At 15 d post infection, the sheath blight disease PDI was significantly lower in the CO1 and CO2 transgenic rice lines, which expressed both chi11 and ap24, than in those which expressed either chi11 or ap24 individually.






Similar content being viewed by others
References
Abad LR, D’Urzo MP, Liu D, Narasimhan ML, Reuveni M, Zhu JK, Niu X, Singh NK, Hasegawa PM, Bressan RA (1996) Antifungal activity of tobacco osmotin has specificity and involves plasma membrane permeabilization. Plant Sci 118:11–23
Anzlovar S, Dalla Serra M, Dermastia M, Menestrina G (1998) Membrane permeabilizing activity of pathogenesis-related protein linusitin from flax seed. Mol Plant-Microbe Interact 11:610–617
Becker-Ritt AB, Carlini CR (2012) Fungitoxic and insecticidal polypeptides. Biopolymers 98:367–384
Brogue K, Chet I, Holliday M, Cressman R, Biddle P, Knowlton S, Mauvais CJ, Broglie R (1991) Transgenic plants with enhanced resistance to the fungal pathogen Rhizoctonia solani. Science 254:1194–1197
Chen WP, Chen PD, Liu DJ, Kynast R, Friebe B, Velazhahan R, Muthukrishnan S, Gill BS (1999) Development of wheat scab symptoms is delayed in transgenic wheat plants that constitutively express a rice thaumatin-like protein gene. Theor Appl Genet 99:755–760
Datta K, Velazhahan R, Oliva N, Ona I, Mew T, Khush GS, Muthukrishnan S, Datta SK (1999) Over-expression of the cloned rice thaumatin-like protein (PR-5) gene in transgenic rice plants enhances environmental friendly resistance to Rhizoctonia solani causing sheath blight disease. Theor Appl Genet 98:1138–1145
Ditta G, Stanfield S, Corbin D, Helinski DR (1980) Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A 77:7347–7351
Fagoaga C, Rodrigo I, Conejero V, Hinarejos C, Tuset JJ, Arnau J, Pina JA, Navarro L, Peña L (2001) Increased tolerance to Phytophthora citrophthora in transgenic orange plants constitutively expressing a tomato pathogenesis related protein PR-5. Mol Breed 7:175–185
Grover A, Pental D (2003) Breeding objectives and requirements for producing transgenics for major field crops of India. Curr Sci 84:310–320
Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42:819–832
Ibeas JI, Lee H, Damsz B, Prasad DT, Pardo JM, Hasegawa PM, Bressan RA, Narasimhan ML (2000) Fungal cell wall phosphomannans facilitate the toxic activity of a plant PR-5 protein. Plant J 23:375–383
Jayaraj J, Punja ZK (2007) Combined expression of chitinase and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar pathogens. Plant Cell Rep 26:1539–1546
Jefferson RA (1987) Assaying chimeric gene in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–404
Kalpana K, Maruthasalam S, Rajesh T, Poovannan K, Kumar KK, Kokiladevi E, Raja JAJ, Sudhakar D, Velazhahan R, Samiyappan R, Balasubramanian P (2006) Engineering sheath blight resistance in elite indica rice cultivars using genes encoding defense proteins. Plant Sci 170:203–215
Kim JK, Jang IC, Wu R, Zuo WN, Boston RS, Lee YH, Ahn IP, Nahm BH (2003) Co-expression of a modified maize ribosome-inactivating protein and a rice basic chitinase gene in transgenic rice plants confers enhanced resistance to sheath blight. Transgenic Res 12:475–484
Kishimoto K, Nishizawa Y, Tabei Y, Hibi T, Nakajima M, Akutsu K (2002) Detailed analysis of rice chitinase gene expression in transgeniccucumber plants showing different levels of disease resistance to gray mold (Botrytis cinerea). Plant Sci 162:655–662
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10:165–174
LaRosa PC, Singh NK, Hasegawa PM, Bressan RA (1989) Stable NaCl tolerance of tobacco cells is associated with enhanced accumulation of osmotin. Plant Physiol 91:855–861
Lin W, Anuratha CS, Datta K, Potrykus I, Muthukrishnan S, Datta SK (1995) Genetic engineering of rice for resistance to sheath blight. Bio/Technology 13:686–691
Liu G, Jia Y, McClung KM, Datta A, Correll JC (2009) Mapping quantitative trait loci responsible for resistance to sheath blight in rice. Phytopathology 99:1078–1084
Liu D, Raghothama KG, Hasegawa PM, Bressan RA (1994) Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci U S A 91:1888–1892
Liu JJ, Stirrock R, Ekramoddoullah AKM (2010) The superfamily of thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Rep 29:419–436
Lorito M, Woo SL, Ambrosio MD, Herman GE, Hayes CK, Kubicek CP, Scala F (1996) Synergistic interaction between cell wall degrading enzymes and membrane affecting compounds. Mol Plant-Microbe Interact 9:206–213
Maruthasalam S, Kalpana K, Kumar KK, Loganathan M, Poovannan K, Raja JAJ, Kokiladevi E, Samiyappan R, Sudhakar D, Balasubramanian P (2007) Pyramiding transgenic resistance in elite indica rice cultivars against the sheath blight and bacterial blight. Plant Cell Rep 26:791–804
Melander M, Kamnert I, Happstadius I, Liljeroth E, Bryngelsson T (2006) Stability of transgene integration and expression in subsequent generations of doubled haploid oilseed rape transformed with chitinase and β-1,3-glucanase genes in a double-gene construct. Plant Cell Rep 25:942–952
Melchers LS, Sela-Buurlage MB, Vloemans SA, Woloshuk CP, Van Roekel JSC, Pen J, Van den Elzen PJM, Cornelissen BJC (1993) Extracellular targeting of the vacuolar tobacco proteins AP24, chitinase and β-1,3-glucanase in transgenic plants. Plant Mol Biol 21:583–593
Moravcikova J, Matusikova I, Libantova J, Bauer M, Mlynarova L (2004) Expression of a cucumber class III chitinase and Nicotiana plumbaginifolia class I glucanase genes in transgenic potato plants. Plant Cell Tissue Organ Cult 79:161–168
Murashige T, Skoog F (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Punja ZK (2006) Recent developments toward achieving fungal disease resistance in transgenic plants. Can J Plant Pathol 28:S298–S308
Rajeevkumar S, Anunanthini P, Sathishkumar R (2015) Epigenetic silencing in transgenic plants. Front Plant Sci 6:1–8
Rao MVR, Parameswari C, Sripriya R, Veluthambi K (2011) Transgene stacking and marker elimination in transgenic rice by sequential Agrobacterium-mediated co-transformation with the same selectable marker gene. Plant Cell Rep 30:1241–1252
Roberts WK, Selitrennikoff CP (1990) Zeamatin, an antifungal protein from maize with membrane-permeabilizing activity. J Gen Microbiol 136:1771–1778
Shah JM, Singh R, Veluthambi K (2013) Transgenic rice lines constitutively co-expressing tlp-D34 and chi11 display enhancement of sheath blight resistance. Biol Plant 57:351–358
Silva J, SchefflerB SY, De Guzman C, Galam D, Farmer A, Woodward J, May G, Oard J (2012) Identification of candidate genes in rice for resistance to sheath blight disease by whole genome sequencing. Theor Appl Gen 124:63–74
Singh NK, Bracker CA, Hasegawa PM, Handa AK, Buckel S, Hermodson MA, Pfankoch E, Regneier FE, Bressan RA (1987) Characterization of osmotin: a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol 85:529–536
Singh NK, Handa AK, Hasegawa PM, Bressan RA (1985) Proteins associated with adaptation to cultured tobacco cells to NaCl. Plant Physiol 79:126–137
Sridevi G, Dhandapani M, Veluthambi K (2005) Agrobacterium-mediated transformation of White Ponni, a non-basmati variety of indica rice (Oryza sativa L.). Current Sci 88:128–132
Sridevi G, Parameswari C, Rajamuni P, Veluthambi K (2006) Identification of hemizygous and homozygous transgenic rice plants in T1 generation by DNA blot analysis. Plant Biotechnol 23:531–534
Sridevi G, Parameswari C, Sabapathi N, Raghupathy V, Veluthambi K (2008) Combined expression of chitinase and β-1,3-glucanase genes in indica rice (Oryza sativa L.) enhances resistance against Rhizoctonia solani. Plant Sci 175:283–290
Sripriya R, Raghupathy V, Veluthambi K (2008) Generation of selectable marker-free sheath blight resistant transgenic rice plants by efficient co-transformation of a cointegrate vector T-DNA and a binary vector T-DNA in one Agrobacterium tumefaciens strain. Plant Cell Rep 27:1635–1644
Subramanyam K, Arun M, Mariashibu TS, Theboral J, Rajesh M, Singh NK, Manickavasagam M, Ganapathi A (2012) Overexpression of tobacco osmotin (Tbosm) in soybean conferred resistance to salinity stress and fungal infections. Planta 236:1909–1925
Swegle M, Kramer KJ, Muthukrishnan S (1992) Properties of barley seed chitinases and release of embryo-associated isoforms during early stages of imbibition. Plant Physiol 99:1009–1014
Velazhahan R, Chen-Cole K, Anuratha CS, Muthukrishnan S (1998) Induction of thaumatin-like proteins (TLPs) in Rhizoctonia solani-infected rice and characterization of two new cDNA clones. Physiol Plant 102:21–28
Vigers AJ, Roberts WK, Selitrennikoff CP (1991) A new family of plant antifungal proteins. Mol Plant-Microbe Interact 4:315–323
Wally O, Jayaraj J, Punja Z (2009) Comparative resistance to foliar fungal pathogens in transgenic carrot plants expressing genes encoding for chitinase, β-1,3-glucanase and peroxidase. Eur J Plant Pathol 123:331–342
Wang Y, Kausch AP, Chandlee JM, Luo H, Ruemmele BA, Browning M, Jackson N, Goldsmith MR (2003) Co-transfer and expression of chitinase, glucanase, and bar genes in creeping bentgrass for conferring fungal disease resistance. Plant Sci 165:497–506
Woloshuk CP, Meulenhoff JS, Sela-Buurlage M, Van Den Elzen PJM, Cornelissen BJC (1991) Pathogen-induced proteins with inhibitory activity towards Phytophthora infestans. Plant Cell 3:619–628
Yabor L, Valle B, Carvajal C, Aragón C, Hernández M, González J, Daquinta M, Arencibia A, Lorenzo JC (2010) Characterization of a field-grown transgenic pineapple clone containing the genes chitinase, AP24, and bar. In Vitro Cell Dev Biol Plant 46:1–7
Zhu B, Chen THH, Li PH (1996) Analysis of late-blight disease resistance and freezing tolerance in transgenic potato plants expressing sense and antisense genes for an osmotin-like protein. Planta 198:70–77
Zuo S, Zhang Y, Chen Z, Jiang W, Feng M, Pan X (2014) Improvement of rice resistance to sheath blight by pyramiding QTLs conditioning disease resistance and tiller angle. Rice Sci 21:318–326
Acknowledgments
We thank Dr. Richard A. Jefferson, Canberra, Australia, for pCAMBIA1301; Dr. Toshihiko Komari, Japan Tobacco, Inc., Shizuoka, Japan, for pSB1; Dr. Stanton B. Gelvin, Purdue University, West Lafayette, Indiana, for Agrobacterium strain LBA4404; Dr. S. Muthukrishnan, Kansas State University, Manhattan, Kansas, for rice chi11 and the chitinase antibody; and Dr. Leo S. Melchers, Mogen International NV, Leiden, The Netherlands, for ap24. We thank Dr. Ray Bressan, Dr. Meena Narasimhan, and Dr. K. G. Raghothama for providing us with the osmotin antibody. We acknowledge Dr. G. Sridevi, Ms. K. Vidhya, and Mr. N. Sabapathi for the construction of pGSD2, pKVD2, and pNSP2, respectively. Program support from the Department of Biotechnology (DBT), Government of India (BT/PR6466/COE/34/16/2012), and the University Grants Commission (UGC), Government of India (No. F. 18-1/2011 [BSR]), BSR Faculty Fellowship to KV are acknowledged. RS is thankful to DBT and UGC for her research fellowships.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Marco Buennostro-Nava
Electronic supplementary material
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Sripriya, R., Parameswari, C. & Veluthambi, K. Enhancement of sheath blight tolerance in transgenic rice by combined expression of tobacco osmotin (ap24) and rice chitinase (chi11) genes. In Vitro Cell.Dev.Biol.-Plant 53, 12–21 (2017). https://doi.org/10.1007/s11627-017-9807-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-017-9807-8