Abstract
Background
Very brief advice (VBA; ≤ 3 min) on quitting is practical and scalable during brief medical interactions with patients who smoke. This study aims to synthesize the effectiveness of VBA for smoking cessation and summarize the implementation strategies.
Methods
We searched randomized controlled trials aiming at tobacco abstinence and comparing VBA versus no smoking advice or no contact from Medline, Embase, CINAHL, Cochrane Library, PsycInfo databases, six Chinese databases, two trial registries ClinicalTrials.gov and WHO-ICTRP from inception to September 30, 2023. Grading of Recommendations, Assessment, Development, and Evaluations framework was used to assess the certainty of the evidence of the meta-analytic findings. The outcomes were self-reported long-term tobacco abstinence at least 6 months after treatment initiation, earlier than 6 months after treatment initiation, and quit attempts. Effect sizes were computed as risk ratio (RR) with 95% CI using frequentist random-effect models.
Data Synthesis
Thirteen randomized controlled trials from 15 articles (n = 26,437) were included. There was moderate-certainty evidence that VBA significantly increased self-reported tobacco abstinence at ≥ 6 months in the adjusted model (adjusted risk ratio ARR 1.17, 95% CI: 1.07–1.27) compared with controls. The sensitivity analysis showed similar results when abstinence was verified by biochemical validation (n = 6 studies, RR 1.53, 95% CI 0.98–2.40). There was high-certainty evidence that VBA significantly increased abstinence at < 6 months (ARR 1.22, 95% CI: 1.01–1.47). Evidence of effect on quit attempts (ARR 1.03, 95% CI 0.97–1.08) was of very low certainty.
Discussion
VBA delivered in a clinical setting is effective in increasing self-reported tobacco abstinence, which provides support for wider adoption in clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
Tobacco cessation prevents premature death, many types of cancers and cardiovascular and pulmonary diseases.1 However, many smokers have not received advice to quit, and only few quit attempters have used tobacco cessation treatment.2,3,4 In low- and middle-income countries, only 40% of smokers received advice to quit smoking from healthcare providers in the past year.2 On the other hand, about 176.8 million adults in 31 countries made a quit attempt in the past 12 months.3 Only about 10% of quit attempters had used tobacco cessation aids, including counseling and medications, in 15 countries,4 probably because of low accessibility or awareness of these services in smokers.5,6
Brief intervention (BI) aims to deliver an evidence-based smoking cessation (SC) intervention within a minimal time period by identifying smokers, advising, and assisting them to quit.7,8 BI was effective in directing patients to SC treatments and increasing tobacco abstinence.9,10,11,12,13 The 5As (Ask, Advise, Assess, Assist, Arrange follow-up) and 5Rs (Relevance, Risk, Rewards, Roadblocks, Repetition) model are the most known BI models recommended by the World Health Organization (WHO),14 the US Centers for Disease Control and Prevention,15 and the 2020 Surgeon General Report.1 However, many healthcare providers cannot adhere to or implement the full BI.16 More simplified SC intervention models, namely very brief advice (VBA), such as AAR (Ask, Advise, Refer),17 AWARD (Ask, Warn, Advice, Refer, Do-it-again),8 and the ABC (Ask about smoking, give Brief advice to quit, and offer Cessation assistance)18 were developed for healthcare practitioners to implement easily in routine medical consultation, with very short duration. While BI acts primarily by motivating quit attempts and delivering quitting aids, VBA acts by giving opportunistic advice to all smokers, irrespective of motivation to quit. For instance, New Zealand’s clinical guideline adopts a proactive “opt-out” ABC approach to provide very brief opportunistic advice to all smokers without a preliminary assessment of willingness to quit.18
Two contextual factors inherent to clinical practice facilitate the implementation of VBA over BI by healthcare providers. First, clinical settings that have time constraints and short consultations may allow only very brief (1–2 min) discussions on smoking cessation. Assessment of motivation as required by “opt-in approaches” is time-consuming and often not feasible.19 Second, not all healthcare professionals are trained or specialized in tobacco cessation and counseling skills to deliver more intensive treatment. Therefore, VBA is probably the most convenient model to advise and refer patients who smoke to use SC services.20
Some randomized controlled trials have tested the effectiveness of VBA) as short as 30 s and supported its effectiveness.21,22 VBA may help reduce the barriers of the increased time demand on healthcare workers in providing SC advice and tackle the impracticality of longer interventions in busy clinical settings. General practitioners trained with the ABC model delivered more SC advice after training than those trained with conventional 5As (between group: 35.7% vs 30.3%, adjusted odds ratio 1.71, 95% CI 0.94–3.12).23 Both healthcare providers and patients preferred VBA for SC due to its feasibility, simplicity, and ease.21,24 BI was commonly defined as taking 10 to 30 min in systematic reviews.10,11,12 Some systematic reviews even included trials testing “BI” which exceeded 30 min,9,13 which far exceeded the usual time (about 10 min) for medical consultation. However, no previous systematic reviews that specifically synthesized the RCTs result in the effectiveness of opportunistic SC advice that was 3 min or less. Thus, we aimed to build upon the previous review by Aveyard et al. (2012) to (i) synthesize the effectiveness of VBA on SC to better assess the specific effect of VBA in 3 min or less without other intensive intervention components and (ii) summarize the implementation strategies and settings of VBA to recognize the contextual factors for implementation.
METHODS
Data Sources and Searches
The study was registered with The International Prospective Register of Systematic Reviews (PROSPERO) (Ref No: CRD42022341466). Based on the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) checklist,25 we elaborated the question of the review in terms of Population, Intervention, Comparison, Outcomes, Study Designs (PICOS), to search systematically: “What is the effectiveness of the VBA on the long-term tobacco abstinence in current (daily or occasional) tobacco users including cigarettes, smokeless tobacco, heated tobacco products, and cigars, compared with no SC advice or no contact from randomized controlled trials?” Medline, Embase, CINAHL, Cochrane Library, PsycInfo databases, two trial registries ClinicalTrials.gov and WHO-ICTRP, and additional six Chinese databases were systematically searched from inception to September 30, 2023. The full search strategy is shown in the eMethods of Supplement 1.
Study Selection
We included studies with the following criteria: (1) individual or cluster randomized controlled trials, (2) long-term tobacco abstinence outcomes were reported, (3) behavioral intervention duration was 3 min or less as stated in manuscripts or judged by assessors, and (4) the control group received no SC advice or no contact. In studies with intervention arm(s) involving pharmacotherapy, we included them if their data from non-pharmacotherapy trial arms can be extracted. We also excluded studies (1) if their number of follow-up interventions was more than twice per month, as such follow-up interventions could have a stronger effect than VBA in the first contact, and (2) recruiting ex-smokers, people currently attempting to quit or non-tobacco users including such as electronic nicotine delivery systems (ENDS). We had no restriction on languages used, but an abstract written in English or Chinese was required. While eligibility of a non-English or non-Chinese full-text was being assessed, a translator would assist in evaluating the study. Eventually, we did not identify any such studies. We had no restrictions on study setting, age limit of the participants, and types of intervention.
Data Extraction and Outcomes
The relevant data were extracted independently by two co-authors (CCCW and WJAH). The primary outcome was self-reported tobacco abstinence at ≥ 6 months after treatment initiation. Assessing posttreatment initiation of abstinence was preferred compared to the target quit day as smokers might or might not have stopped smoking on the target quit day.26 We used the commonly adopted minimal time of follow-up for the assessment of tobacco cessation, which is 6 months after intervention. Continuous abstinence was used if both continuous and point prevalence results were available. Self-report smoking status was chosen over biochemical validation of abstinence because some RCTs did not validate abstinence with biochemical methods. Also, the process of inviting smokers to come back for biochemical validation could have motivated some to quit smoking for a few days before validation and often includes monetary incentives, which could increase abstinence. Nevertheless, we performed a sensitivity analysis to examine whether the pooled estimates of self-reported abstinence produced similar results as those verified by biochemical validation.
Secondary outcomes included tobacco abstinence at < 6 months after treatment initiation and quit attempts. “Quit attempts” was defined as at least one attempt to stop using tobacco products lasting for 24 h or more.27 Risk ratios were extracted when available or calculated based on the reported descriptive statistics.
Quality Assessment
We used the GRADE approach to evaluate the overall certainty of evidence for key outcomes based on study risk of bias, inconsistency of results, indirectness of evidence, imprecision, and publication bias.28 Sensitivity analyses were done by moderation analysis based on the risk of bias obtained by the Cochrane Risk of Bias Assessment Tool, particularly the items related to the exchangeability of treatment and control group, random sequence generation, and allocation concealment. Inconsistency was assessed by Cochran’s Q test in the overall estimate and test of heterogeneity in subgroup analysis. Indirectness was assessed by whether the outcome of interest was measured differently from recommendations or guidelines. Imprecision was assessed by the effect estimate in relation to the null effect.28 A funnel plot was used to assess publication bias with Egger’s regression and significance level of P < 0.1.29 When publication bias was detected, we further adjusted for the missing studies by non-prespecified Duval and Tweedie’s “trim and fill” analysis and estimated the numbers and outcomes of missing studies.30
Data Synthesis
Clinical heterogeneities were assessed by tabulating the study characteristics, including delivery settings (e.g., inpatient, outpatient, community), interventionists (i.e., profession to deliver the intervention), use of intervention models (i.e., any specific advice model adopted in the study), duration of the advice, use of self-help materials, and any relevant features where available.
Two co-authors (CCCW and WJAH) evaluated the risk of bias independently for the included studies using the Cochrane collaboration risk of bias tool.31 Any discrepancies were resolved by discussion with a third co-author (DCYT). A stacked bar chart was used to show the categories of (1) high risk, (2) unclear risk, (3) low risk in each domain included in the risk of bias tool.
To check the comparability of the studies before pooling the outcome data in the meta-analysis, we conducted tests for moderation to assess the potential influence of differences in randomization generation, conflict of interest, and follow-up time points across studies. When there is no evidence of moderation from these factors, the data from the included studies were pooled to generate a robust estimate of the overall treatment effect.
Quantitative Analysis
Data analyses were done by R, with “metafor” package.32 We used random-effect frequentist meta-analytic models to analyse the tobacco abstinence outcomes, with a significance level of P < 0.05. We used the inverse variance weighting method to pool the result of the combined study effect for risk ratios and 95% confidence interval (CI) when available. The number needed to treat (NNT) was estimated by the reciprocal of the risk difference. The results were visualized by a forest plot, showing weights and publication year, individual and pooled effect estimates and 95% CI.
Subgroup Analysis
We did subgroup analyses on certain study characteristics of age, high- vs low-income countries and types of interventionists when there were more than two available studies. We used Cochran’s Q test to examine the heterogeneity quantitatively with a significance level of P < 0.1.33 The I-squared statistics by Higgins and Thompson were used to quantify heterogeneity.34
RESULTS
Study and Participant Characteristics
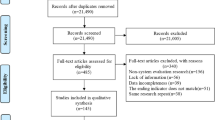
A total of 13 RCTs (no cluster RCTs) from 15 articles (n = 26,437) published from 1979 to 2021 were included in the synthesis35,36,37,38,39,40,41,42,43,44,45,46,47,48,49 (Fig. 1). The extracted general information and abstinence results in each study are presented in Tables 1 and 2, respectively. More details are also shown in eResults 1.
Intervention Strategies
Twelve studies recruited subjects in outpatient clinics (n = 10)35,36, 38,39,40, 42,43,44,45,46,47, 49 and emergency departments (n = 2),37,41 and one study did not specify the setting.48 Interventions were mainly delivered by physicians (n = 11)35,36,37,38,39,40, 42,43,44,45,46, 48, 49 and the others by dentists (n = 1)47 or nurses (n = 1).41 In all studies, the intervention duration was 3 min or less. One study took 2 to 3 min,44 5 took 1 to 2 min,39,40, 45, 46, 48 3 took about 1 min,35,36, 41, 49 and 3 took no more than 30 s.37,38, 42, 43 One study did not state the exact duration, but we regarded it as less than 3 min because the advice was described as only including tobacco harm on oral health and involved no intensive counseling by dentists.47
Ten studies standardized the intervention in an advice script (n = 6)37,38, 41,42,43, 48, 49 or guided by a clear protocol (n = 4)35,36, 39, 44, 47 and three studies mentioned that interventionists advised patients using their own style without a clearer intervention protocol.40,45, 46 In addition to verbal advice, 10 studies incorporated written materials such as leaflets and small cards.35,36,37,38, 40, 41, 44,45,46,47,48 One study delivered a “starter kit” including non-nicotine gums and rubber bands.47 Three studies included boosters of telephone follow-up after patients received the VBA.38,41, 49 One study included in-person clinic visit boosters.44
Eight studies included workshops or briefings about the intervention protocol to build capacity of VBA interventionists,35,36, 38, 41,42,43,44, 47,48,49 whereas three studies reported the workshops only took less than an hour.38,42, 43, 48 The remaining five studies did not report the VBA training for the interventionists.
Risk of Bias
Six studies clearly mentioned the methods of randomization and concealment.35,36,37,38, 41,42,43, 49 No studies blinded participants or personnel about the intervention. Attrition bias was either low or unclear. Outcome reporting bias was mostly unclear or high. Considerable heterogeneity of methods was found specifically on selection bias and other bias. Details on assessing the risk of bias are shown in eResults 2.
We found no significant moderation by randomization generation, conflict of interest, and follow-up time points on the abstinence outcomes, supporting that an overall analysis integrating the available data was appropriate. Regarding quit attempts, we found that the heterogeneity of random sequence generation and operationalization of quit attempts moderated the quit attempt outcome; hence, three studies with high risk of bias were removed when pooling the risk ratio. Details on assessing the moderation effect of the study characteristics are shown in eResults 3.
Effectiveness of Outcomes
Publication bias was detected in abstinence assessed at ≥ 6 months and < 6 months. A trim and fill analysis was also conducted by imputing hypothetical studies to correct the funnel plot to be symmetrical (see eFigure 2a, b, 3a and b). The crude average treatment effect for abstinence assessed at ≥ 6 months was RR 1.28 (95% CI 1.10–1.49; NNT 66, 95%CI 46–112; Fig. 2a). After adjusting publication bias, the average treatment effect of VBA for tobacco abstinence at ≥ 6 months was small and significant (RR 1.17, 95% CI 1.07–1.27; NNT 73, 95%CI 49–143; Fig. 2b). The average treatment effect for biochemically validated abstinence from six studies was not significant, with a greater RR and wider confidence interval than self-reported abstinence (RR 1.53, 95% CI 0.98–2.40; NNT 256, 95%CI 135–2855; Fig. 3). The average treatment effect for abstinence at < 6 months was RR 1.35 (95% CI: 1.15–1.58; NNT 72, 95%CI 51–122; eFigure 4). After adjusting publication bias, the average treatment effect for tobacco abstinence at < 6 months was small and significant (RR 1.22, 95% CI: 1.01–1.47; NNT 82, 95%CI 55–166; in eFigure 5).
The average treatment effect from five studies for quit attempts was small and significant (RR 1.18, 95% CI: 1.02–1.35; eFigure 6). After excluding studies with high selection bias from pooled analysis, the average treatment effect for quit attempts was nearly null (RR 1.03, 95% CI: 0.97–1.08; eFigure 7).
Subgroup analysis on setting was not conducted because all included studies were done in clinical settings, except one study, which did not specify the setting. Also, the available studies did not report findings stratified by age subgroups. Analysis was only conducted on five studies with the subgroup aged 18 years and older. Moreover, only two studies with a low risk of bias reported quit attempts, thereby subgroup analysis was not done. Most subgroup analyses for age, economic status of countries, interventionists, control interventions, and length of advice showed significant intervention effects of VBA on abstinence outcomes at ≥ 6 months and < 6 months (eFigures 8–17). The intervention effect of VBA in low- and middle-income countries (i.e., China only, excluding Hong Kong) on abstinence at ≥ 6 months was moderate but not significant (eFigure 10: RR 1.57, 95%CI 0.89–2.78). When the advice length was 1–2 min, the effect was small and not significant (eFigure 14: RR 1.28, 95%CI 0.98–1.67).
Certainty of Evidence
Our GRADE approach showed that the certainty of evidence for the treatment effect on abstinence at ≥ 6 months and < 6 months was moderate and high, respectively, but the evidence was of very low certainty for the treatment effect on quit attempts (eResults 4).
DISCUSSION
The current review supplemented the review by Aveyard et al. (2012), which included very brief and brief interventions by including newer and more focused evidence to assess the effectiveness of VBA in 3 min or less. This world’s first meta-analysis on VBA showed that VBA, delivered in 3 min or less by healthcare professionals, effectively increased self-reported abstinence at ≥ 6 months by 17% and < 6 months by 22% compared to no SC advice, with moderate and high certainty of evidence respectively, but quit attempts showed very low certainty evidence.
The included studies showed variability in methodological rigor. For example, we found unclear random sequence generation and treatment concealment in a few studies. Our sensitivity analyses showed no significant differences in the treatment effect across studies with different methods. Also, integrating RCTs of lower quality in the meta-analysis did not lead to an inflation of the treatment effect size for increasing abstinence. Therefore, the variation in study methods did not compromise our conclusion on the effectiveness of VBA.
Our subgroup analyses on the treatment effect by age, economic status of countries, interventionists, and length of advice showed no significant moderation effect. The meta-analysis in high-income countries showed a significant treatment effect on increasing abstinence, whereas such an effect in low- or middle-income countries was not significant, probably because of the small sample size. Our analysis included only three studies in China, which is an upper middle-income country; hence, more RCTs on the effectiveness of VBA, especially in other low- or middle-income countries, are warranted.
The outcome of quit attempts could be extracted from only five studies. Most of the studies did not consistently define a meaningful quit attempt, as the length of abstinence varied from 24 h to 7 days. As the evidence had very low certainty, the findings should be interpreted cautiously. The true effect could in fact be higher, given that quit attempts are a pre-requisite of eventual abstinence. More precise measures and consistent, high-quality evidence are needed to show stronger certainty of the effectiveness of quit attempts.
We showed that VBA had a smaller effect size than brief intervention and medication. Hence, VBA should not replace or reduce the delivery of other effective behavioral interventions when the latter are feasible and available. If the settings can facilitate longer consultations with the patients or have sufficient capacity to deliver intensive treatment, VBA only may not optimize the quitting outcomes. Healthcare professionals need to evaluate all the contextual factors and incorporate appropriate service models for smoking cessation.
Our qualitative synthesis of different VBA implementation strategies highlighted a few features which can be included in future VBA guidelines. Firstly, in three studies, clinicians only needed brief training of about an hour or less before delivering the intervention.35,36, 38, 41,42,43,44, 47,48,49 Since healthcare professionals already have extensive knowledge about the harms of tobacco and benefits of quitting, future training should emphasize the effectiveness and operation of VBA. They can certainly save lives and prevent serious smoking-induced diseases by spending little time and effort; even most smokers would not succeed quickly. Secondly, clear and specific advice models (e.g., 2A1R model, ABC model, AWARD model) conceptualize what the key components in a VBA, and these models should help health professionals understand what the “must-do” advice is. Even if they are not familiar with these models, they can simply warn about the high mortality due to smoking, that one out of two smokers will be killed by smoking,50,51,52 advise to quit as soon as possible and refer. Last, other quitting support such as referral to SC services when available, printed resources, and follow-up boosters (e.g., phone calls) can be incorporated into VBA when some smokers want more quitting support, but the evidence of these additional efforts is unclear.
LIMITATIONS
The study had several limitations. First, the variety of the selected RCTs was limited. There is lack of RCTs testing the effectiveness of VBA in community-based settings, such as community health centers and health promotional campaigns. Only two RCTs included nurses or dentists as interventionists. Most studies predominantly recruited cigarette smokers and very few included other tobacco products. Hence, our findings have limited generalizability in these areas. Second, treating self-reported smoking abstinence as primary outcome without biochemical validation is another limitation of this review. Over-reporting of quitting is possible when using self-report alone, which may lead to inaccurate effect size estimates and biased results if over-reporting is not evenly distributed between intervention and control groups. Third, we did not require the presence of SC services as a selection criterion, because the present study aimed to test the effectiveness of offering VBA, regardless of using SC services or support following the delivery of VBA. We found that four studies indicating the availability of local smoking cessation services.38,41,42,43 Such information in other studies was not reported, so we could not ascertain if these services were really not available, and subgroup analysis of this feature would not yield reliable results. Last, 10 out of the 13 included studies did not incorporate any validation of interventionist compliance with the intervention protocol. For advice intended to be delivered in just a few minutes without validating compliance, the effects measured might overestimate the true impact of the advice. Future research needs to incorporate objective mechanisms to assess whether very brief advice is adequately delivered by interventionists and determine the actual “dose” and duration of the intervention delivered.
CONCLUSIONS
This study showed that VBA had a significant although with a small effect size in increasing abstinence assessed at ≥ 6 months or < 6 months after treatment initiation. Our finding supports a call of action on delivering VBA in all contacts to patients who smoke in clinical settings. The simplicity, low cost, and high reach level of VBA intervention supports a wider implementation to further increase tobacco abstinence.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Smoking Cessation: A Report of the Surgeon General. US Department of Health and Human Services. 2020. Available from: https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf. Accessed 24 Apr 2024.
Owusu D, Wang KS, Quinn M, Aibangbee J, John RM, Mamudu HM. Health Care Provider Intervention and Utilization of Cessation Assistance in 12 Low- and Middle-Income Countries. Nicotine Tob Res. 2019;21(2):188-96.
Ahluwalia IB, Tripp AL, Dean AK, Mbulo L, Arrazola RA, Twentyman E, et al. Tobacco Smoking Cessation and Quitline Use Among Adults Aged ≥15 Years in 31 Countries: Findings From the Global Adult Tobacco Survey. Am J Prev Med. 2021;60(3 Suppl 2):S128-s35.
Borland R, Li L, Driezen P, Wilson N, Hammond D, Thompson ME, et al. Cessation assistance reported by smokers in 15 countries participating in the International Tobacco Control (ITC) policy evaluation surveys. Addiction. 2012;107(1):197-205.
World Health Organization. WHO report on the global tobacco epidemic, 2023: protect people from tobacco smoke. 2023.
Piné-Abata H, McNeill A, Murray R, Bitton A, Rigotti N, Raw M. A survey of tobacco dependence treatment services in 121 countries. Addiction. 2013;108(8):1476-84.
West R, Raw M, McNeill A, Stead L, Aveyard P, Bitton J, et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110(9):1388-403.
Wang MP, Suen YN, Li WH, Lam CO, Wu SY, Kwong AC, et al. Intervention With Brief Cessation Advice Plus Active Referral for Proactively Recruited Community Smokers: A Pragmatic Cluster Randomized Clinical Trial. JAMA Intern Med. 2017;177(12):1790-7.
Akanbi MO, Carroll AJ, Achenbach C, O'Dwyer LC, Jordan N, Hitsman B, et al. The efficacy of smoking cessation interventions in low- and middle-income countries: a systematic review and meta-analysis. Addiction. 2019;114(4):620-35.
Aveyard P, Begh R, Parsons A, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction. 2012;107(6):1066-73.
Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013;2013(5):Cd000165.
Rice VH, Heath L, Livingstone-Banks J, Hartmann-Boyce J. Nursing interventions for smoking cessation. Cochrane Database Syst Rev. 2017;12(12):Cd001188.
Wray JM, Funderburk JS, Acker JD, Wray LO, Maisto SA. A Meta-Analysis of Brief Tobacco Interventions for Use in Integrated Primary Care. Nicotine Tob Res. 2018;20(12):1418-26.
Toolkit for delivering the 5A’s and 5R’s brief tobacco interventions in primary care. 2014. Report No.: 9241506954.
Centers for Disease Control and Prevention. Smoking Cessation: Fast Facts [Internet]. 2020. Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html. Accessed 24 Apr 2024.
Bartsch AL, Härter M, Niedrich J, Brütt AL, Buchholz A. A Systematic Literature Review of Self-Reported Smoking Cessation Counseling by Primary Care Physicians. PLoS One. 2016;11(12):e0168482.
Patwardhan PD, Chewning BA. Ask, advise and refer: hypothesis generation to promote a brief tobacco-cessation intervention in community pharmacies. Int J Pharm Pract. 2009;17(4):221-9.
McCormack J, Walker N, McRobbie H, Wright K, Nosa V, Fernandes B, et al. Revised Guidelines for smoking cessation in New Zealand, 2021. N Z Med J. 2022;135(1558):54-64.
Kastaun S, Leve V, Hildebrandt J, Funke C, Becker S, Lubisch D, et al. Effectiveness of training general practitioners to improve the implementation of brief stop-smoking advice in German primary care: study protocol of a pragmatic, 2-arm cluster randomised controlled trial (the ABCII trial). BMC Fam Pract. 2019;20:1-18.
Kotz D. Implementation of a new'opt-out'default for tobacco treatment is urgently needed, but requires free access to evidence-based treatments. Addiction (Abingdon, England). 2015;110(3):387-8.
Papadakis S, Anastasaki M, Papadakaki M, Antonopoulou Μ, Chliveros C, Daskalaki C, et al. 'Very brief advice' (VBA) on smoking in family practice: a qualitative evaluation of the tobacco user's perspective. BMC Fam Pract. 2020;21(1):121.
Caponnetto P, Maglia M, Floresta D, Ledda C, Vitale E, Polosa R, et al. A randomized controlled trial to compare group motivational interviewing to very brief advice for the effectiveness of a workplace smoking cessation counseling intervention. J Addict Dis. 2020;38(4):465-74.
Kastaun S, Leve V, Hildebrandt J, Funke C, Klosterhalfen S, Lubisch D, et al. Training general practitioners in the ABC versus 5As method of delivering stop-smoking advice: a pragmatic, two-arm cluster randomised controlled trial. ERJ Open Res. 2021;7(3):00621-2020. https://doi.org/10.1183/23120541.00621-2020.
van Schayck OCP, Bindels L, Nijs A, van Engelen B, van den Bosch A, Muller IS, et al. The experience of general practitioners with Very Brief Advice in the treatment of tobacco addiction. NPJ Prim Care Respir Med. 2020;30(1):40.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Piper ME, Bullen C, Krishnan-Sarin S, Rigotti NA, Steinberg ML, Streck JM, et al. Defining and Measuring Abstinence in Clinical Trials of Smoking Cessation Interventions: An Updated Review. Nicotine Tob Res. 2020;22(7):1098-106.
Starr G, Rogers T, Schooley M, Porter S, Wiesen E, Jamison N. Key outcome indicators for evaluating comprehensive tobacco control programs. Atlanta, GA: Centers for Disease Control and Prevention. 2005.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-94.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629-34.
Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-63.
Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. https://doi.org/10.1002/9781119536604.ch8
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1-48.
Tate MW, Brown SM. Note on the Cochran Q test. J Am Stat Assoc. 1970;65(329):155-60.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21(11):1539-58.
Betson C, Lam T, Chung T, Chung S, editors. A randomized controlled trial of smoking cessation in Government out-patient clinics in Hong Kong. Tobacco: The Growing Epidemic: Proceedings of the Tenth World Conference on Tobacco or Health, 24–28 August 1997, Beijing, China. 2000. Springer.
Lam TH, Chung TWH, Betson CL, Chung SF. A randomized controlled trial of smoking cessation in Government out-patient clinics in Hong Kong. In: Dissemination Reports of Health Services Research funded by Hospital Authority. 2000.
Cheung KW, Wong IW, Fingrut W, Tsai APY, Ke SR, Shojaie S, et al. Randomized controlled trial of emergency department initiated smoking cessation counselling and referral to a community counselling service. Canadian Journal of Emergency Medicine. 2018;20(4):556-64.
Cheung YTD, Jiang N, Jiang CQ, Zhuang RS, Gao WH, Zhou J, et al. Physicians' very brief (30-sec) intervention for smoking cessation on 13 671 smokers in China: a pragmatic randomized controlled trial. Addiction. 2021;116(5):1172-85.
Folsom AR, Grimm RH, Jr. Stop smoking advice by physicians: a feasible approach? Am J Public Health. 1987;77(7):849-50.
Jamrozik K, Vessey M, Fowler G, Wald N, Parker G, Van Vunakis H. Controlled trial of three different antismoking interventions in general practice. Br Med J (Clin Res Ed). 1984;288(6429):1499-503.
Li WHC, Ho KY, Wang MP, Cheung DYT, Lam KKW, Xia W, et al. Effectiveness of a Brief Self-determination Theory-Based Smoking Cessation Intervention for Smokers at Emergency Departments in Hong Kong: A Randomized Clinical Trial. JAMA Intern Med. 2020;180(2):206-14.
Lin PR, Zhao ZW, Cheng KK, Lam TH. The effect of physician's 30 s smoking cessation intervention for male medical outpatients: a pilot randomized controlled trial. J Public Health (Oxf). 2013;35(3):375-83.
Lin PRZ. The effect of physician’s brief smoking cessation advice for male outpatients: a pilot randomized controlled trial. Guangzhou Medical College Master’s Dissertation. 2012. (in Chinese) 医生非常简短戒烟警告对门诊吸烟男病人 戒烟及减少吸烟的作用:随机对照临床试验. 广州医学院 硕士学位论文. 2012.
Loke AY, Lam TH. A randomized controlled trial of the simple advice given by obstetricians in Guangzhou, China, to non-smoking pregnant women to help their husbands quit smoking. Patient education and counseling. 2005;59(1):31-7.
Russell MA, Wilson C, Taylor C, Baker CD. Effect of general practitioners' advice against smoking. Br Med J. 1979;2(6184):231-5.
Russell MA, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general practitioner's advice against smoking. Br Med J (Clin Res Ed). 1983;287(6407):1782-5.
Severson HH, Andrews JA, Lichtenstein E, Gordon JS, Barckley MF. Using the hygiene visit to deliver a tobacco cessation program: results of a randomized clinical trial. J Am Dent Assoc. 1998;129(7):993-9.
Slama K, Redman S, Perkins J, Reid AL, Sanson-Fisher RW. The effectiveness of two smoking cessation programmes for use in general practice: a randomised clinical trial. Bmj. 1990;300(6741):1707-9.
Wu L, He Y, Jiang B, Zhang D, Tian H, Zuo F, et al. Very brief physician advice and supplemental proactive telephone calls to promote smoking reduction and cessation in Chinese male smokers with no intention to quit: a randomized trial. Addiction. 2017;112(11):2032-40.
Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519.
World Health Organization. WHO report on the global tobacco epidemic, 2008: the MPOWER package. 2008.
Peto R. Smoking and death: the past 40 years and the next 40. BMJ. 1994;309(6959):937-9.
Acknowledgements:
We thank the World Health Organization for supporting the study. The study was previously presented in SRNT 2023, March 1-4.
Funding
World Health Organization (PROSPERO: CRD42022341466).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest:
The authors declare that they do not have a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, C.C.W., He, W.J.A., Gouda, H. et al. Effectiveness of Very Brief Advice on Tobacco Cessation: A Systematic Review and Meta-Analysis. J GEN INTERN MED 39, 1721–1734 (2024). https://doi.org/10.1007/s11606-024-08786-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-024-08786-8