Abstract
Background
The effect of clinical interventions may vary by patients’ frailty status. Understanding treatment effect heterogeneity by frailty could lead to frailty-guided treatment strategies and reduce overtreatment and undertreatment. This systematic review aimed to examine the effect modification by frailty in randomized controlled trials (RCTs) that evaluate pharmacological, non-pharmacological, and multicomponent interventions.
Methods
We searched PubMed, Web of Science, EMBASE, and ClinicalTrial.gov, from their inception to 8 December 2023. Two reviewers independently extracted trial data and examined the study quality with senior authors.
Results
Sixty-one RCTs that evaluated the interaction between frailty and treatment effects in older adults were included. Frailty was evaluated using different tools such as the deficit accumulation frailty index, frailty phenotype, and other methods. The effect of several pharmacological interventions (e.g., edoxaban, sacubitril/valsartan, prasugrel, and chemotherapy) varied according to the degree of frailty, whereas other treatments (e.g., antihypertensives, vaccinations, osteoporosis medications, and androgen medications) demonstrated consistent benefits across different frailty levels. Some non-pharmacological interventions had greater benefits in patients with higher (e.g., chair yoga, functional walking, physical rehabilitation, and higher dose exercise program) or lower (e.g., intensive lifestyle intervention, psychosocial intervention) levels of frailty, while others (e.g., resistance-type exercise training, moderate-intensive physical activity, walking and nutrition or walking) produced similar intervention effects. Specific combined interventions (e.g., hospital-based disease management programs) demonstrated inconsistent effects across different frailty levels.
Discussion
The efficacy of clinical interventions often varied by frailty levels, suggesting that frailty is an important factor to consider in recommending clinical interventions in older adults.
Registration
PROSPERO registration number CRD42021283051.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
Frailty is a clinical state of reduced physiologic reserve and increased vulnerability to poor health outcomes. The prevalence of frailty is 12 to 24% in community-dwelling older adults1 and almost 50% in hospitalized patients2 and nursing home residents.3 In the United States, 15% of the older population was frail and 45% was pre-frail.4 Those with frailty are predisposed to adverse health events, including falls, disability, dementia, hospitalization, institutionalization in long-term care, and death.5, 6 Compared with a robust group, pre-frail and frail older adults incurred more healthcare costs.7
Clinicians increasingly consider frailty in treatment decision-making due to its association with poor treatment outcomes.1 Nonetheless, our understanding on how the benefits and risks of clinical interventions vary by patients’ frailty remains limited. A more nuanced understanding of treatment effect heterogeneity by frailty could lead to frailty-guided treatment strategies and reduce overtreatment and undertreatment, which could lead to improved health outcomes, better quality of life, and more targeted use of healthcare resources. For instance, a robust patient might tolerate a more aggressive treatment regimen potentially leading to improved disease control or even cure. In contrast, a frail patient with depleted physiologic reserve might benefit more from a less invasive, more supportive approach focused on symptom management. To answer this question, frailty subgroup analyses of randomized controlled trials (RCTs) are increasingly conducted to investigate treatment effect heterogeneity by frailty.8
This systematic review was conducted to synthesize the findings from RCTs that assessed treatment effects stratified by participants’ frailty levels. We examined how frailty was assessed in RCTs and whether the efficacy and safety of interventions varied by frailty category.
METHODS
We followed the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) statement to conduct a systematic review.9 The protocol was registered in the PROSPERO international prospective register of systematic reviews (registration number CRD42021283051, https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=283051) on 2 November 2021.
Our review addressed a key question: Did frailty modify intervention effects in RCTs of pharmacological, non-pharmacological, and multicomponent interventions?
Data Sources and Searches
A literature search of PubMed, Web of Science, and EMBASE was performed. ClinicalTrial.gov was also searched to identify related trials. Our search included articles published up until December 8, 2023, and we restricted our search to articles published in English. Two authors (JZ, LG) independently reviewed titles, abstracts, and full-text articles to identify eligible studies. Any discrepancies were discussed and resolved with another author (AY). Moreover, we reviewed reference lists of relevant articles and studies potentially meeting our inclusion criteria to minimize retrieval bias. In certain instances, authors indicated in their papers that frailty modifies intervention effects but did not present these findings in tables, figures, or supplemental files. We made attempts to contact all such authors to collect the relevant data.
Study Selection
We included studies that involved older adults; encompassed RCTs that utilized pharmacological, non-pharmacological, or multicomponent interventions; were published in English; and stratified participants by frailty or conducted an interaction effect or subgroup analysis of frailty and the intervention. We excluded protocols, reviews, editorials, narrative reviews, case reports, case series, animal studies, duplicate publications, and articles without an available full text. Furthermore, studies that did not measure frailty or use it as a stratification variable were also excluded.
Data Extraction and Quality Assessment
We extracted the following variables from each study: first author’s name, year of publication, country of study, patient characteristics (number and disease types), intervention type (pharmacological, non-pharmacological, and multicomponent), control measures, frailty measurements, frailty sub-groups, endpoints, and the modifying effects of frailty. The extracted data were organized into three tables based on the frailty measurement tools used (i.e., deficit accumulation frailty index [range, 0 to 1; higher scores indicate more severe frailty], frailty phenotype [robust, pre-frail, and frail categories], and other frailty assessments). Two authors (JZ, LG) conducted this abstraction by reviewing each article. Any disagreements were resolved through discussion with senior authors (AY, DHK).
Cochrane Risk of Bias Tool for randomized trials was used to assess the included studies.10 The following domains were included: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, and other sources of bias. Two authors (LG, JZ) independently evaluated each domain as having a high, low, or unclear risk of bias. Conflicts were resolved by discussion with another author (AY).
Data Synthesis and Analysis
We did not perform meta-analysis due to heterogeneity of frailty tools and intervention types. Instead, we qualitatively synthesized the findings from studies based on the frailty tools used: trials that used (1) deficit accumulation frailty index, (2) frailty phenotype, or (3) other frailty measurement tools. Studies within each category were further grouped into intervention types, which included pharmacological, non-pharmacological, or multicomponent intervention. Two authors (JZ, LG) independently and in duplicate rated the certainty of evidence and resolved disagreements by discussion and consultation with senior authors (AY, DHK).
Role of the Funding Source
This study was supported by the National Institute on Aging of the National Institutes of Health. The funding source had no role in the design, collection, analysis, or interpretation of the data, or the decision to submit the manuscript for publication.
RESULTS
Study Selection and Characteristics of the Included Trials
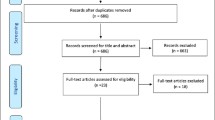
Figure 1 details study selection. Our database searches yielded 5917 references, of which 61 articles11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71 met the inclusion criteria. The included trials are summarized by the type of frailty assessment: frailty index (24 trials) (Table 1), frailty phenotype (17 trials) (Table 2), and other assessments (20 trials) (Table 3). Further details of each trial can be found in Appendix Table. The mean age of trial populations ranged from 58.711 to 87.112 years and the proportion of women ranged from 23.429 to 93.3%14 (in particular, one RCT was 100% male15 and the other 100% female16). There were 26 trials evaluating pharmacological interventions (sample size 4017 to 31,98918), 27 trials on non-pharmacological interventions (sample size 3014, 18 to 5145),11 and 8 trials on multicomponent interventions (sample size 17319 to 1464).20
Quality of the Included Trials
Most of the included articles have low likelihood of bias. Risk of bias related to blinding of participants and personnel and incomplete outcome data were identified in 18 and 12 trials, respectively. The risk of bias for each included trial is shown in both the Appendix Figure and Table.
Evaluation of Treatment Effect by Deficit Accumulation Frailty Index
Pharmacologic Interventions
The included 15 trials assessed whether the effect of the following treatments was different by frailty levels: anticoagulants,21 antihypertensives,22,23,24,25,26 heart failure (HF) medication,27,28,29,30,31 anti-interleukin 1 monoclonal antibody,13 and vaccinations.32,33,34
Anticoagulants
In a trial of 20,867 adults with atrial fibrillation conducted over 34 months across 46 countries,21 edoxaban was associated with lower rates of major bleeding compared with warfarin in patients with frailty index 0.12 to <0.36 (edoxaban 60 mg) and patients with frailty index <0.36 (edoxaban 30 mg), but not in patients with frailty index <0.12 (edoxaban 60 mg) and frailty index 0.36 to <1.0 (edoxaban 30 mg or 60 mg) (p-for-interaction=not reported [NR]). There was a significant treatment effect of edoxaban compared with warfarin on stroke or systemic embolism, but the treatment effect was not different across the frailty spectrum (p-for-interaction=NR).
Antihypertensives
There was a significant treatment effect of antihypertensive medication reduction compared with usual care on systolic blood pressure control,22 indapamide ± perindopril compared with placebo on stroke and cardiovascular events.24 However, there was no significant treatment effect of intensive systolic blood pressure control compared with standard control on syncope and falls,23 indapamide ± perindopril compared with placebo on all-cause mortality24. Furthermore, five trials22,23,24,25,26 have concluded that the effects of antihypertensive medication reduction,22 intensive blood pressure control,23, 25, 26 and indapamide ± perindopril24 compared with control treatment, were similar across frailty levels for cardiovascular events,24, 25 stroke,24 all-cause mortality,24, 25 systolic blood pressure control,22 change in systolic blood pressure,22 syncope,23 hypotension,23 falls,23 and cerebral blood flow.26
HF Medications
Sacubitril/valsartan was associated with lower rates of HF hospitalization or cardiovascular death (p-for-interaction = 0.002) and HF hospitalization (p-for-interaction = 0.001) in patients with a higher frailty index (0.311 to 1), but not in patients with frailty index <0.311.28 In a trial of 4742 patients with HF with reduced ejection fraction and elevated natriuretic peptide,29 dapagliflozin was associated with lower rates of cardiovascular death (p-for-interaction=NR) and all-cause death (p-for-interaction=NR) compared with placebo in patients with frailty index ≤0.210, but not in patients with frailty index >0.210. Besides, dapagliflozin was associated with lower rates of cardiovascular outcome (p-for-interaction=NR) compared with placebo in patients with frailty index ≤0.210 or frailty index ≥0.311,29, 31 but not in patients with frailty 0.210 to <0.311. Another trial found dapagliflozin was associated with lower rates of HF hospitalization or cardiovascular death (p-for-interaction=NR) compared with placebo in patients with frailty index <0.311, but not in patients with frailty index ≥0.311.30 There was significant treatment effect of spironolactone compared with placebo27 on HF hospitalization or cardiovascular death, but the treatment effect was not different across frailty spectrum (p-for-interaction = 0.40).
Vaccinations
There was significant treatment effect of adjuvanted recombinant zoster vaccine compared with placebo,32 23-valent polysaccharide vaccine compared with 23-valent polysaccharide vaccine with 7-valent pneumococcal conjugate vaccine on serotype 19F IgG (mg/mL)34. There was no significant treatment effect of 23-valent polysaccharide vaccine compared with 23-valent polysaccharide vaccine with 7-valent pneumococcal conjugate vaccine on serotype 4 IgG (mg/mL) and serotype 18C IgG (mg/mL)33. Vaccine efficacy does not differ across frailty levels for two specific interventions (p-for-interaction=NR): adjuvanted recombinant zoster vaccine compared with placebo32 and 23-valent polysaccharide vaccine compared with 23-valent polysaccharide vaccine with 7-valent pneumococcal conjugate vaccine.33, 34
Anti-interleukin 1 Monoclonal Antibody
Canakinumab had a significant treatment effect on incident major adverse cardiovascular events compared with placebo, but the effect was not different across frailty levels (p-for-interaction=NR).13
Non-pharmacological Intervention
Six trials assessed whether the effect of following treatment was different by frailty levels: diabetes management,11 physical activity and exercise,12, 35, 37, 38 and other.36
Diabetes Management
In a trial of 5145 adults with type 2 diabetes and overweight or obesity conducted over 118 months in the US,11 intensive lifestyle intervention was associated with lower cardiovascular events compared with diabetes support and education in patients with frailty index <0.178, not in patients with frailty index ≥0.178 (p-for-interaction = 0.01).
Physical Activity and Exercise
Increasing frailty index was associated with greater reductions in pain score (p-for-interaction = 0.02) and pain interference (p-for-interaction = 0.01) with chair yoga compared with health education.35 Aerobic exercise training was associated with lower rates of composite of all-cause hospitalization or all-cause mortality compared with usual care in patients with frailty index >0.21, but not in patients with frailty index ≤0.21 (p-for-interaction=NR).37 Similarly, physical activity was associated with lower rates of major mobility disability compared with health education in patients with frailty index ≥0.15, but not in patients with frailty index <0.15 (p-for-interaction=NR)38. There was significant treatment effect of intensive exercise compared with usual care on mortality, but the effect was not different across the frailty spectrum (p-for-interaction=NR).12
Other
In a trial conducted in the Netherlands,36 involving 3092 adults aged 60 and older over a 12-month follow-up, there was evidence to suggest that the effect of frailty screening alone or when combined with a nurse-led care program compared with usual care was beneficial on the modified Katz-15 function score, but the effect was different across the frailty spectrum (p-for-interaction=NR).
Multicomponent Intervention
We identified three trials that assessed the effects of multicomponent interventions among frailty subgroups.
Geriatric Assessment
In a trial of 541 patients with incurable cancer and impairment in one or more geriatric assessment domains,39 the effect of geriatric assessment compared with usual care on conversations (p-for-interaction = 0.611), concerns acknowledged (p-for-interaction = 0.740), and concerns addressed (p-for-interaction = 0.940) was beneficial, but the effect was similar across the frailty spectrum.
Pharmacist-Led Deprescribing Intervention
In a trial of 363 older adults living in the community conducted over 6 months in the US, the effect of pharmacist-led deprescribing intervention compared with usual care on changes in drug burden index 0.5 or more, anticholinergic cognitive burden was beneficial, but the effect was not different across the frailty spectrum (p-for-interaction=NR).40, 41
Evaluation of Treatment Effect by Frailty Phenotype
Pharmacology Intervention
Four trials assessed whether the effect of the following treatments was different by frailty levels: antiplatelet medications,42 anticoagulants,43 osteoporosis medications,16 and androgen medications.15
Antiplatelet Medications
In a trial42 conducted across 52 countries with 9326 adults aged 65 or older suffering from unstable angina, prasugrel was associated with higher rates of the composite outcome of cardiovascular death, myocardial infarction, or stroke compared to clopidogrel in patients with one to two components of the frailty phenotype, but not in patients with zero or three to five components of frailty phenotype (p-for-interaction = 0.032).
Other
The remaining three interventions (including anticoagulants,43 osteoporosis medications,16 and androgen medications)15 were beneficial, but all concluded that intervention effect was not different across the frailty spectrum. Specifically, the effect of edoxaban, strontium, or testosterone gel compared with placebo on stroke or systemic embolism (p-for-interaction = 0.55), all-cause death (p-for-interaction = 0.06), net clinical composite outcome (p-for-interaction = 0.42), vertebral fracture (p-for-interaction = 0.11), and isometric knee extension peak torque (p-for-interaction = 0.68) was not different across the frailty spectrum.
Non-pharmacological Intervention
The included 10 trials assessed whether the effects of the following treatments were different by frailty levels: diabetes management44 and physical activity and exercise.14, 37, 45, 46, 47, 49, 50, 51, 52
Diabetes Management
In a trial conducted44 by Rodriguez-Manas in 2019 across 7 European countries, involving 964 adults aged over 70 with type 2 diabetes mellitus and functional impairment, multimodal intervention was compared with usual care over a 12-month follow-up. The results indicated diabetes management was beneficial, but no evidence of a differential effect of the multimodal intervention on Short Physical Performance Battery (SPPB) score across the frailty spectrum (p-for-interaction = 0.49).
Physical Activity and Exercise
There was significant effect of functional walking compared with usual pattern of activities on persistent mobility disability,46 physical rehabilitation intervention compared with attention control on SPPB score.45,46 The majority of the trials revealed no significant differences across the frailty spectrum concerning the effects of various physical interventions. These interventions, when compared to controls or usual care, consistently improved outcomes such as physical performance,37, 47 Mini-BESTest score,14 functional gait assessment,14 SPPB score,47 days at home,49 dominant handgrip strength,50 major mobility disability,51 persistent mobility disability51, skeletal muscle mass index,52 insulin-like growth factor,52 dehydroepiandrosterone sulfate,52 and 25-hydroxy vitamin D52 (p-for-interaction = NR or >0.05). Exceptions to this pattern were identified in two areas: (1) functional walking interventions were associated with higher risks of falls in patients with three to five components of the frailty phenotype compared to usual activities (p-for-interaction = 0.002);46 (2) physical rehabilitation intervention was associated with greater improvement in SPPB score in patients with three to five components of the frailty phenotype (p-for-interaction = 0.03).37
Multicomponent Intervention
We identified three trials that assessed the effects of multicomponent interventions among frailty subgroups.
Multifactorial, Interdisciplinary Intervention
Two articles53, 54 from the same RCT illustrated varying effects of multifactorial, interdisciplinary interventions (details shown in the footnote of Table 2) compared to usual care on patients with three to five components of the frailty phenotype, but not in patients with zero to two components of the frailty phenotype. A significant association between multidomain intervention and usual care was observed with greater Life Space Assessment score in patients with three components of the frailty phenotype at 3 months (p-for-interaction = 0.03), but not in patients with four to five components of the frailty phenotype and this effect attenuated by 12 months (p-for-interaction = 0.4). There was an increase in gait speed in patients with four to five components of the frailty phenotype at 12 months, but not in patients with three components of the frailty phenotype (p-for-interaction = 0.03). No significant differences were found in the effects of the intervention on SPPB and Physiological Profile Assessment across the frailty spectrum (p-for-interaction=NR).
Multidomain Intervention and Omega-3 Polyunsaturated Fatty Acids (n3 PUFA)
In the trial20 conducted by Tabue-teguo in 2018 in France, involving three different interventions (multicomponent + n3 PUFA vs multicomponent alone vs n3 PUFA alone, details shown in the footnote of Table 2) on a population of 1680 older adults over a 3-year follow-up, partial measures of cognitive function (Trail-Making Test A and B) showed significant improvement. However, no significant differences in cognitive function across the frailty spectrum were found (p-for-interaction > 0.05).
Evaluation of Treatment Effect by Other Frailty Tools
Of the included trials, 20 trials measured frailty using other frailty assessment tools, for example, INTERMED for the Elderly Self-Assessment (INTERMED-E-SA),55, 56 Groningen Frailty Indicator (GFI),36, 55,56,57 frailty-associated conditions,18 modified frailty score,19 two tests of physical abilities,58 simplified Eastern Cooperate Oncology Group (ECOG)–based frailty assessment,59, 60 International Myeloma Working Group geriatric score,17, 61, 62 and the Study of Osteoporotic Fractures frailty index.63
Pharmacology Intervention
Seven trials assessed whether the effect of the following treatments were different by frailty levels: anti-neoplastic therapy17, 59,60,61,62,64 and vaccinations.18
Anti-neoplastic Therapy for Multiple Myeloma
Six trials assessed the effects of various treatments on progression-free survival (PFS) and overall survival (OS) in patients with different frailty levels. Four trials found that their interventions, including melphalan-prednisone-lenalidomide,59 ixazomib,62 and lenalidomide and dexamethasone57 were potentially associated with more prolonged PFS and OS in fit patients or patients with lower levels of frailty. Two trials found significant effect of lenalidomide compared to placebo,17 or daratumumab plus lenalidomide/dexamethasone compared with lenalidomide/dexamethasone58 on PFS58 or OS,17 but the effect was not different across the frailty spectrum (measured by International Myeloma Working Group geriatric score, simplified ECOG-based frailty assessment, or frailty assessment based on four components, separately) (p-for-interaction=NR in all trials).
Vaccinations
There was significant treatment effect of high-dose inactivated influenza vaccine compared with a standard-dose vaccine on laboratory-confirmed influenza, but the treatment effect was not different across the frailty spectrum measured by frailty-associated conditions (details shown in the footnotes of Table 3) (p-for-interaction = 0.838).18
Non-pharmacological Intervention
The included 11 trials assessed whether the effects of the following treatment were different by frailty levels: radiation therapy,65 surgical procedures,66 physical activity and exercise,63, 67, 68 psychosocial intervention,69 and others.55, 56, 58, 70, 71
Radiation Therapy
There was no significant treatment effect of 1-week course radiation therapy compared with a 3-week course on OS,65 and the effect was not different across the frailty spectrum measured by the Karnofsky Performance Status (p-for-interaction=NR).
Surgical Procedures
There was no significant treatment effect of anterior minimally invasive hemiarthroplasty compared with lateral Hardinge hemiarthroplasty on timed up and go duration,66 and the impact was not different across the frailty spectrum measured by the Functional Independence Measure, Charlson Index, and Medication score (p-for-interaction=NR).
Physical Activity and Exercise
A higher dose exercise program led to a greater improvement in health-related quality of life compared to no exercise in patients meeting at least two of three frailty assessments (including Combined Fried frailty phenotype, modified Physical Performance Test, and 7-point Clinical Frailty Scale) (p-for-interaction = 0.037).67 Additionally, physical activity was associated with higher total cardiovascular event rates compared to health education in patients with an SPPB score of 8 to 9, but not in those with an SPPB score <8 (p-for-interaction = 0.006).68 However, there was no evidence that the effect of physical activity compared with health education on 400-m gait-speed, 4-m gait-speed was different across the frailty spectrum (p-for-interaction=NR).63
Psychosocial Intervention
One psychosocial intervention was associated with improvement in instrumental activities of daily living (p-for-interaction < 0.01), potential enhancement in physical performance (p-for-interaction = 0.02), and a reduction in mortality (p-for-interaction = 0.01) in patients with a summary frailty index ≤3, but not in patients with a summary frailty index 4 to 5 (details shown in the footnotes of Table 3). However, these effects were not observed in patients with a frailty index of 4–5, indicating that the benefits of psychosocial intervention may be specific to lower levels of frailty.69
Others
No evidence that the effects of various interventions (for HF, stroke, physical frailty, or others), such as home telemedicine,71 home-based physiotherapy,58 and embrace program (details shown in the footnotes of Table 3),55, 56 differed across the frailty spectrum for non-fatal HF events, function measures, and various domains of health and well-being (p-for-interaction=NR or >0.05). However, a home-based intervention program was associated with a lower disability score compared to an educational program in patients meeting one of two frailty assessments (rapid gait test and chair stand test), but not in patients meeting both tests (p-for-interaction < 0.001 at 7 months and p-for-interaction = 0.005 at 12 months).58 Additionally, embrace was linked to greater improvements in quality of life in patients with INTERMED-E-SA ≥ 16, and patients with INTERMED-E-SA <16 and GFI 5 to 15, but not in patients with INTERMED-E-SA 16 to 20, or INTERMED-E-SA<16 and GFI <5 (p-for-interaction=NR).55, 56
Multicomponent Intervention
We identified two trials19, 57 that assessed the effect of multicomponent interventions among frailty subgroups.
Hospital-Based Disease Management Programs (DMP)
In one RCT19, 173 older patients with HF were randomly assigned to DMP or the usual care group. During a 2-year follow-up, a statistically significant effect of DMP compared to usual care was found in lowering all-cause admissions in patients with a higher modified frailty score, but not in patients with a lower frailty score (p-for-interaction = 0.018). However, the effect on death and/or HF admissions was not different across the frailty spectrum (p-for-interaction = 0.208).
Prevention of Care (POC) Approach
Metzelthin57 compared the POC approach with usual care in community dwelling frail older people and found no treatment effect on the Groningen Activity Restriction Scale, and the effect was not different across frailty subgroups measured by GFI (p-for-interaction > 0.05).
DISCUSSION
We found several RCTs that examined whether the effects of pharmacological, non-pharmacological, and multicomponent interventions varied by frailty levels as measured using a deficit accumulation frailty index, frailty phenotype, or other frailty tools. For most pharmacological interventions including flu vaccines, the effect was consistent across frailty levels with a few exceptions: some interventions (e.g., edoxaban,21 prasugrel,42 chemotherapy for multiple myeloma59, 61, 62) were more effective or safer in robust patients, while the benefit of sacubitril/valsartan28 was greater in frail patients. Intensive lifestyle changes11 and exercise interventions14, 35, 44, 45, 47, 49,50,51,52, 67 seem to benefit frail older adults as much as or more than non-frail older adults. The effect of complex, multicomponent interventions depended on the type of the intervention. A multicomponent intervention delivered by an interdisciplinary team of physiotherapists, a dietician, a geriatrician, a rehabilitation physician, and a nurse resulted in a greater improvement in life space and gait speed among patients with frailty.53, 54 Similarly, a DMP for HF patients led to a greater reduction in all-cause admissions in frail patients.19 In contrast, frailty screening in routine primary care settings36, 57 or a multicomponent program including n3 PUFA supplementation in patients with memory complaints20 failed to demonstrate the benefit compared to usual care, regardless of frailty levels. Psychosocial support was more effective in functional recovery after stroke in less frail patients.69
The evaluation of treatment effect heterogeneity by patients’ frailty levels offers great potential to allow healthcare providers to optimize medical interventions in older adults. In scenarios where frailty significantly modifies treatment effects, healthcare providers can individualize the delivery of medical interventions that maximizes the benefit and minimizes the risks based on patients’ frailty levels. A common misconception in practice is that having frailty is conflated with decreased treatment efficacy, which may lead to under-treatment of frail patients as demonstrated by the delayed uptake of newly approved medications.72,73,74 By showing that treatment benefits do not vary by frailty status, under-treatment of frail patients can be avoided. Likewise, over-treatment can be minimized for frail patients near the end-of-life who may not gain benefit from treatments.
In applying the findings of our review to fully realize the potential of frailty-guided clinical management, there are important caveats to consider.8 First, the adoption of a standardized, validated frailty assessment tool is essential. Frailty assessment tools used in RCTs were often created post hoc using the items only available in the trial settings or were modified from the original tool, resulting in measurement error (i.e., misclassifying patients’ frailty levels). It also limits the applicability of the study findings. Adoption of a brief, standardized, validated frailty screening is imperative to translate the frailty-specific effects from RCTs into clinical practice. The Clinical Frailty Scale is a clinical judgment–based assessment that can be completed within 3 min and has been validated in various clinical settings, including primary care, emergency department, inpatient, and preoperative settings.75, 76 Second, post hoc analysis of RCTs should be considered hypothesis generating until further independent confirmation. Secondary analysis of RCTs that is not pre-specified is subject to confounding, type I error (false positive findings from multiple testing), and type II error (false negative findings due to inadequate power). Future adequately powered RCTs are needed to test for heterogeneity of treatment effects by the spectrum of frailty. Third, enrolling more older adults with frailty in RCTs is needed. Given the challenges in recruiting patients with frailty and assessing frailty in RCTs, pragmatic clinical trial or hybrid effectiveness and implementation study design in routine care settings should be considered. Lastly, the mechanisms by which frailty influences response to treatment are unknown. Further research is warranted to elucidate the biological mechanisms. Moreover, it remains uncertain whether improving frailty prior to treatment can change response to treatments by enhancing the benefits and mitigating the harms associated with treatments.
Limitations
Our review has a few limitations. Despite our effort to identify RCTs that assessed the frailty subgroup-specific treatment effect in multiple electronic databases, we did not include publications in non-English literature and the studies that did not report frailty-specific effect estimates, raising the possibility of publication bias. The heterogeneity of frailty assessment tools, type of interventions, and outcomes precluded meta-analysis.
CONCLUSION
This systematic review of post hoc analysis of RCTs suggests that the effectiveness and safety of certain pharmacological interventions can vary according to patients’ frailty levels and that the lifestyle or exercise interventions may benefit patients with frailty as much as or more than those without frailty. Although this preliminary evidence supports the potential utility of frailty assessment before treatment decisions, it also reveals the current challenges in delivering frailty-guided clinical care based on the post hoc analyses of RCTs. We call for further investigation into frailty-specific treatment effects by adopting a validated frailty assessment in RCTs of medical interventions involving older adults.
References
O’Caoimh R, Sezgin D, O’Donovan MR, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50(1):96-104. https://doi.org/10.1093/ageing/afaa219.
Doody P, Asamane EA, Aunger JA, et al. The prevalence of frailty and pre-frailty among geriatric hospital inpatients and its association with economic prosperity and healthcare expenditure: A systematic review and meta-analysis of 467,779 geriatric hospital inpatients. Ageing Res Rev. 2022;80:101666. https://doi.org/10.1016/j.arr.2022.101666.
Kojima G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc. 2015;16(11):940-945. https://doi.org/10.1016/j.jamda.2015.06.025.
Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in Older Adults: A Nationally Representative Profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70(11):1427-1434. https://doi.org/10.1093/gerona/glv133.
Chu W, Chang SF, Ho HY. Adverse Health Effects of Frailty: Systematic Review and Meta-Analysis of Middle-Aged and Older Adults With Implications for Evidence-Based Practice. Worldviews Evid Based Nurs. 2021;18(4):282-289. https://doi.org/10.1111/wvn.12508.
Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc. 2016;17(12):1163.e1-1163.e17. https://doi.org/10.1016/j.jamda.2016.09.010.
Chi J, Chen F, Zhang J, et al. Impacts of frailty on health care costs among community-dwelling older adults: A meta-analysis of cohort studies. Arch Gerontol Geriatr. 2021;94:104344. https://doi.org/10.1016/j.archger.2021.104344.
Kim D, Zhong L, Rich MW. Frailty-Guided Management of Cardiovascular Disease—From Clinical Trials to Clinical Practice. JAMA Cardiology. 2023;8(10):897-8.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928.
Simpson FR, Pajewski NM, Beavers KM, et al. Does the Impact of Intensive Lifestyle Intervention on Cardiovascular Disease Risk Vary According to Frailty as Measured via Deficit Accumulation? Melzer D, ed. J Gerontol Ser A. 2021;76(2):339-345. https://doi.org/10.1093/gerona/glaa153.
Pérez-Zepeda MU, Martínez-Velilla N, Kehler DS, Izquierdo M, Rockwood K, Theou O. The impact of an exercise intervention on frailty levels in hospitalised older adults: secondary analysis of a randomised controlled trial. Age Ageing. 2022;51(2):afac028. https://doi.org/10.1093/ageing/afac028.
Orkaby AR, Thomson A, MacFadyen J, et al. Effect of canakinumab on frailty: A post hoc analysis of the CANTOS trial. Aging Cell. Published online November 5, 2023:e14029. https://doi.org/10.1111/acel.14029.
Gomes GCV, Simões MDS, Lin SM, et al. Feasibility, safety, acceptability, and functional outcomes of playing Nintendo Wii Fit PlusTM for frail older adults: A randomized feasibility clinical trial. Maturitas. 2018;118:20-28. https://doi.org/10.1016/j.maturitas.2018.10.002.
Srinivas-Shankar U, Roberts SA, Connolly MJ, et al. Effects of Testosterone on Muscle Strength, Physical Function, Body Composition, and Quality of Life in Intermediate-Frail and Frail Elderly Men: A Randomized, Double-Blind, Placebo-Controlled Study. J Clin Endocrinol Metab. 2010;95(2):639–650. 2016092613080600917.
Rolland Y, Van Kan GA, Gillette-Guyonnet S, Roux C, Boonen S, Vellas B. Strontium ranelate and risk of vertebral fractures in frail osteoporotic women. Bone. 2011;48(2):332-338. https://doi.org/10.1016/j.bone.2010.08.022.
Brioli A, Manz K, Pfirrmann M, et al. Frailty impairs the feasibility of induction therapy but not of maintenance therapy in elderly myeloma patients: final results of the German Maintenance Study (GERMAIN). J Cancer Res Clin Oncol. 2020;146(3):749-759. https://doi.org/10.1007/s00432-019-03101-z.
DiazGranados CA, Dunning AJ, Robertson CA, Talbot HK, Landolfi V, Greenberg DP. Efficacy and immunogenicity of high-dose influenza vaccine in older adults by age, comorbidities, and frailty. Vaccine. 2015;33(36):4565-4571. https://doi.org/10.1016/j.vaccine.2015.07.003.
Pulignano G, Del Sindaco D, Di Lenarda A, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med. 2010;11(10):739-747. https://doi.org/10.2459/JCM.0b013e328339d981.
Tabue-Teguo M, Barreto de Souza P, Cantet C, et al. Effect of Multidomain Intervention, Omega-3 Polyunsaturated Fatty Acids Supplementation or their Combinaison on Cognitive Function in Non-Demented Older Adults According to Frail Status: Results from the MAPT Study. J Nutr Health Aging. 2018;22(8):923-927. https://doi.org/10.1007/s12603-018-1024-6.
Wilkinson C, Wu J, Searle SD, et al. Clinical outcomes in patients with atrial fibrillation and frailty: insights from the ENGAGE AF-TIMI 48 trial. BMC Med. 2020;18(1):401. https://doi.org/10.1186/s12916-020-01870-w.
Sheppard JP, Burt J, Lown M, et al. Effect of Antihypertensive Medication Reduction vs Usual Care on Short-term Blood Pressure Control in Patients With Hypertension Aged 80 Years and Older: The OPTIMISE Randomized Clinical Trial. JAMA. 2020;323(20):2039. https://doi.org/10.1001/jama.2020.4871.
Sink KM, Evans GW, Shorr RI, et al. Syncope, Hypotension, and Falls in the Treatment of Hypertension: Results from the Randomized Clinical Systolic Blood Pressure Intervention Trial: SYNCOPE, HYPOTENSION, AND FALLS IN THE SPRINT TRIAL. J Am Geriatr Soc. 2018;66(4):679-686. https://doi.org/10.1111/jgs.15236.
Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13(1):78. https://doi.org/10.1186/s12916-015-0328-1.
Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged ≥75 Years: A Randomized Clinical Trial. JAMA. 2016;315(24):2673. https://doi.org/10.1001/jama.2016.7050.
Dolui S, Detre JA, Gaussoin SA, et al. Association of Intensive vs Standard Blood Pressure Control With Cerebral Blood Flow: Secondary Analysis of the SPRINT MIND Randomized Clinical Trial. JAMA Neurol. 2022;79(4):380-389. https://doi.org/10.1001/jamaneurol.2022.0074.
Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial: Frailty in HFpEF. Eur J Heart Fail. 2018;20(11):1570-1577. https://doi.org/10.1002/ejhf.1308.
Butt JH, Dewan P, Jhund PS, et al. Sacubitril/Valsartan and Frailty in Patients With Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2022;80(12):1130-1143. https://doi.org/10.1016/j.jacc.2022.06.037.
Butt JH, Dewan P, Merkely B, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Heart Failure With Reduced Ejection Fraction : A Post Hoc Analysis of the DAPA-HF Trial. Ann Intern Med. 2022;175(6):820-830. https://doi.org/10.7326/M21-4776.
Butt JH, Jhund PS, Belohlávek J, et al. Efficacy and Safety of Dapagliflozin According to Frailty in Patients With Heart Failure: A Prespecified Analysis of the DELIVER Trial. Circulation. 2022;146(16):1210-1224. https://doi.org/10.1161/CIRCULATIONAHA.122.061754.
Vart P, Butt JH, Jongs N, et al. Efficacy and Safety of Dapagliflozin in Patients with Chronic Kidney Disease across the Spectrum of Frailty. J Gerontol A Biol Sci Med Sci. Published online August 1, 2023:glad181. https://doi.org/10.1093/gerona/glad181.
Curran D, Kim JH, Matthews S, et al. Recombinant Zoster Vaccine Is Efficacious and Safe in Frail Individuals. J Am Geriatr Soc. 2021;69(3):744-752. https://doi.org/10.1111/jgs.16917.
MacIntyre CR, Ridda I, Gao Z, et al. A Randomized Clinical Trial of the Immunogenicity of 7-Valent Pneumococcal Conjugate Vaccine Compared to 23-Valent Polysaccharide Vaccine in Frail, Hospitalized Elderly. Borrow R, ed. PLoS ONE. 2014;9(4):e94578. https://doi.org/10.1371/journal.pone.0094578.
MacIntyre CR, Ridda I, Trent MJ, McIntyre P. Persistence of immunity to conjugate and polysaccharide pneumococcal vaccines in frail, hospitalised older adults in long-term follow up. Vaccine. 2019;37(35):5016-5024. https://doi.org/10.1016/j.vaccine.2019.07.005.
Park J, Sherman DG, Agogo G, Hoogendijk EO, Liu Z. Frailty modifies the intervention effect of chair yoga on pain among older adults with lower extremity osteoarthritis: Secondary analysis of a nonpharmacological intervention trial. Exp Gerontol. 2020;134:110886. https://doi.org/10.1016/j.exger.2020.110886.
Bleijenberg N, Drubbel I, Schuurmans MJ, et al. Effectiveness of a Proactive Primary Care Program on Preserving Daily Functioning of Older People: A Cluster Randomized Controlled Trial. J Am Geriatr Soc. 2016;64(9):1779-1788. https://doi.org/10.1111/jgs.14325.
Pandey A, Segar MW, Singh S, et al. Frailty Status Modifies the Efficacy of Exercise Training Among Patients With Chronic Heart Failure and Reduced Ejection Fraction: An Analysis From the HF-ACTION Trial. Circulation. 2022;146(2):80-90. https://doi.org/10.1161/CIRCULATIONAHA.122.059983.
Quach J, Theou O, Pérez-Zepeda MU, Godin J, Rockwood K, Kehler DS. Effect of a physical activity intervention and frailty on frailty trajectory and major mobility disability. J Am Geriatr Soc. 2022;70(10):2915-2924. https://doi.org/10.1111/jgs.17941.
Gilmore N, Xu H, Kehoe L, et al. Evaluating the association of frailty with communication about aging-related concerns between older patients with advanced cancer and their oncologists. Cancer. 2022;128(5):1101-1109. https://doi.org/10.1002/cncr.34010.
Jamieson H, Nishtala PS, Bergler HU, et al. Deprescribing Anticholinergic and Sedative Drugs to Reduce Polypharmacy in Frail Older Adults Living in the Community: A Randomized Controlled Trial. J Gerontol A Biol Sci Med Sci. 2023;78(9):1692-1700. https://doi.org/10.1093/gerona/glac249.
Nishtala PS, Pickering JW, Bergler U, Mangin D, Hilmer SN, Jamieson H. Post Hoc Analyses of a Randomized Controlled Trial for the Effect of Pharmacist Deprescribing Intervention on the Anticholinergic Burden in Frail Community-Dwelling Older Adults. J Am Med Dir Assoc. 2023;24(8):1253-1260. https://doi.org/10.1016/j.jamda.2023.05.014.
White HD, Westerhout CM, Alexander KP, et al. Insights from the TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Eur Heart J. 2016;5(3):231-242.
Akashi S, Oguri M, Ikeno E, et al. Outcomes and Safety of Very-Low-Dose Edoxaban in Frail Patients With Atrial Fibrillation in the ELDERCARE-AF Randomized Clinical Trial. JAMA Netw Open. 2022;5(8):e2228500. https://doi.org/10.1001/jamanetworkopen.2022.28500.
Rodriguez‐Mañas L, Laosa O, Vellas B, et al. Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle. 2019;10(4):721-733. https://doi.org/10.1002/jcsm.12432.
Coelho-Júnior HJ, Uchida MC. Effects of Low-Speed and High-Speed Resistance Training Programs on Frailty Status, Physical Performance, Cognitive Function, and Blood Pressure in Prefrail and Frail Older Adults. Front Med. 2021;8:702436. https://doi.org/10.3389/fmed.2021.702436.
Faber MJ, Bosscher RJ, Chin A Paw MJ, van Wieringen PC. Effects of Exercise Programs on Falls and Mobility in Frail and Pre-Frail Older Adults: A Multicenter Randomized Controlled Trial. Arch Phys Med Rehabil. 2006;87(7):885-896. https://doi.org/10.1016/j.apmr.2006.04.005.
Kitzman DW, Whellan DJ, Duncan P, et al. Physical Rehabilitation for Older Patients Hospitalized for Heart Failure. N Engl J Med. 2021;385(3):203-216. https://doi.org/10.1056/NEJMoa2026141.
Pandey A, Kitzman DW, Nelson MB, et al. Frailty and Effects of a Multidomain Physical Rehabilitation Intervention Among Older Patients Hospitalized for Acute Heart Failure: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2023;8(2):167-176. https://doi.org/10.1001/jamacardio.2022.4903.
Suikkanen SA, Soukkio PK, Aartolahti EM, et al. Effects of Home-Based Physical Exercise on Days at Home and Cost-Effectiveness in Pre-Frail and Frail Persons: Randomized Controlled Trial. J Am Med Dir Assoc. 2021;22(4):773-779. https://doi.org/10.1016/j.jamda.2020.06.005.
Tieland M, Verdijk LB, De Groot LCPGM, Van Loon LJC. Handgrip Strength Does Not Represent an Appropriate Measure to Evaluate Changes in Muscle Strength During an Exercise Intervention Program in Frail Older People. Int J Sport Nutr Exerc Metab. 2015;25(1):27-36. https://doi.org/10.1123/ijsnem.2013-0123.
Trombetti A, Hars M, Hsu FC, et al. Effect of Physical Activity on Frailty: Secondary Analysis of a Randomized Controlled Trial. Ann Intern Med. 2018;168(5):309. https://doi.org/10.7326/M16-2011.
Yamada M, Nishiguchi S, Fukutani N, Aoyama T, Arai H. Mail-Based Intervention for Sarcopenia Prevention Increased Anabolic Hormone and Skeletal Muscle Mass in Community-Dwelling Japanese Older Adults: The INE (Intervention by Nutrition and Exercise) Study. J Am Med Dir Assoc. 2015;16(8):654-660. https://doi.org/10.1016/j.jamda.2015.02.017.
Fairhall N, Sherrington C, Kurrle SE, Lord SR, Lockwood K, Cameron ID. Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: randomised controlled trial. BMC Med. 2012;10(1):120. https://doi.org/10.1186/1741-7015-10-120.
Fairhall N, Sherrington C, Lord SR, et al. Effect of a multifactorial, interdisciplinary intervention on risk factors for falls and fall rate in frail older people: a randomised controlled trial. Age Ageing. 2014;43(5):616-622. https://doi.org/10.1093/ageing/aft204.
Spoorenberg SLW, Wynia K, Uittenbroek RJ, Kremer HPH, Reijneveld SA. Effects of a population-based, person-centred and integrated care service on health, wellbeing and self-management of community-living older adults: A randomised controlled trial on Embrace. Evans CJ, ed. PLoS ONE. 2018;13(1):e0190751. https://doi.org/10.1371/journal.pone.0190751.
Uittenbroek RJ, Kremer HPH, Spoorenberg SLW, Reijneveld SA, Wynia K. Integrated Care for Older Adults Improves Perceived Quality of Care: Results of a Randomized Controlled Trial of Embrace. J Gen Intern Med. 2017;32(5):516-523. https://doi.org/10.1007/s11606-016-3742-y.
Metzelthin SF, van Rossum E, de Witte LP, et al. Effectiveness of interdisciplinary primary care approach to reduce disability in community dwelling frail older people: cluster randomised controlled trial. BMJ. 2013;347(sep10 7):f5264-f5264. https://doi.org/10.1136/bmj.f5264.
Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A Program to Prevent Functional Decline in Physically Frail, Elderly Persons Who Live at Home. N Engl J Med. 2002;347(14):1068-1074. https://doi.org/10.1056/NEJMoa020423.
Facon T, Dimopoulos MA, Meuleman N, et al. A simplified frailty scale predicts outcomes in transplant-ineligible patients with newly diagnosed multiple myeloma treated in the FIRST (MM-020) trial. Leukemia. 2020;34(1):224-233. https://doi.org/10.1038/s41375-019-0539-0.
Facon T, Cook G, Usmani SZ, et al. Daratumumab plus lenalidomide and dexamethasone in transplant-ineligible newly diagnosed multiple myeloma: frailty subgroup analysis of MAIA. Leukemia. 2022;36(4):1066-1077. https://doi.org/10.1038/s41375-021-01488-8.
Bringhen S, D’Agostino M, Paris L, et al. Lenalidomide-based induction and maintenance in elderly newly diagnosed multiple myeloma patients: updated results of the EMN01 randomized trial. Haematologica. 2020;105(7):1937-1947. https://doi.org/10.3324/haematol.2019.226407.
Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127(9):1102-1108. https://doi.org/10.1182/blood-2015-08-662627.
Custodero C, Agosti P, Anton SD, et al. Effect of Physical Activity Intervention on Gait Speed by Frailty Condition: A Randomized Clinical Trial. J Am Med Dir Assoc. 2023;24(4):489-496. https://doi.org/10.1016/j.jamda.2023.01.023.
Dimopoulos MA, Špička I, Quach H, et al. Ixazomib as Postinduction Maintenance for Patients With Newly Diagnosed Multiple Myeloma Not Undergoing Autologous Stem Cell Transplantation: The Phase III TOURMALINE-MM4 Trial. J Clin Oncol. 2020;38(34):4030-4041. https://doi.org/10.1200/JCO.20.02060.
Guedes De Castro D, Matiello J, Roa W, et al. Survival Outcomes With Short-Course Radiation Therapy in Elderly Patients With Glioblastoma: Data From a Randomized Phase 3 Trial. Int J Radiat Oncol. 2017;98(4):931-938. https://doi.org/10.1016/j.ijrobp.2017.03.037.
Saxer F, Studer P, Jakob M, et al. Minimally invasive anterior muscle-sparing versus a transgluteal approach for hemiarthroplasty in femoral neck fractures-a prospective randomised controlled trial including 190 elderly patients. BMC Geriatr. 2018;18(1):222. https://doi.org/10.1186/s12877-018-0898-9.
Kaushal N, Langlois F, Desjardins-Crépeau L, Hagger MS, Bherer L. Investigating dose–response effects of multimodal exercise programs on health-related quality of life in older adults. Clin Interv Aging. 2019; 14:209-217. https://doi.org/10.2147/CIA.S187534.
Newman AB, Dodson JA, Church TS, et al. Cardiovascular Events in a Physical Activity Intervention Compared With a Successful Aging Intervention: The LIFE Study Randomized Trial. JAMA Cardiol. 2016;1(5):568. https://doi.org/10.1001/jamacardio.2016.1324.
Ertel KA, Glymour MM, Glass TA, Berkman LF. Frailty modifies effectiveness of psychosocial intervention in recovery from stroke. Clin Rehabil. 2007;21(6):511-522. https://doi.org/10.1177/0269215507078312.
Gladman J, Forster A, Young J. Hospital- and Home-based Rehabilitation after Discharge from Hospital for Stroke Patients: Analysis of Two Trials. Age Ageing. 1995;24(1):49-53. https://doi.org/10.1093/ageing/24.1.49.
Comín-Colet J, Enjuanes C, Verdú-Rotellar JM, et al. Impact on clinical events and healthcare costs of adding telemedicine to multidisciplinary disease management programmes for heart failure: Results of a randomized controlled trial. J Telemed Telecare. 2016;22(5):282-295. https://doi.org/10.1177/1357633X15600583.
Ko D, Lin KJ, Bessette LG, et al. Trends in Use of Oral Anticoagulants in Older Adults With Newly Diagnosed Atrial Fibrillation, 2010-2020. JAMA Netw Open. 2022;5(11):e2242964. https://doi.org/10.1001/jamanetworkopen.2022.42964.
Ko D, Pande A, Lin KJ, et al. Utilization of P2Y12 Inhibitors in Older Adults With ST-Elevation Myocardial Infarction and Frailty. Am J Cardiol. 2023;207:245-252. https://doi.org/10.1016/j.amjcard.2023.08.059.
Lee YC, Lin JK, Ko D, et al. Frailty and uptake of angiotensin receptor neprilysin inhibitor for heart failure with reduced ejection fraction. J Am Geriatr Soc. 2023;71(10):3110-3121. https://doi.org/10.1111/jgs.18481.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ Can Med Assoc J J Assoc Medicale Can. 2005;173(5):489-495. https://doi.org/10.1503/cmaj.050051.
Rockwood K, Theou O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can Geriatr J CGJ. 2020;23(3):210-215. https://doi.org/10.5770/cgj.23.463.
Acknowledgements:
We appreciate assistance and feedback from David Kho, MD; Jinxin Zhang, MSN; Grace Garcia, MHA, BSN; Chan Mi Park, MD, MPH; and Pin-Hsien Wu, MD.
Funding
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Numbers R01AG071809 and K24AG073527 to Dr. Kim.
Author information
Authors and Affiliations
Contributions
Aaron Yao has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy for the data analysis.
• Study concept and design: Aaron Yao, Dae Hyun Kim.
• Acquisition, analysis, and interpretation of data: Linhui Gao, Jiajun Zhang, Aaron Yao, Dae Hyun Kim.
• Drafting of the manuscript: Linhui Gao, Aaron Yao, Jiajun Zhang.
• Critical revision of the manuscript for important intellectual content: Aaron Yao, Dae Hyun Kim; Joyce M. Cheng, Linhui Gao, Jiajun Zhang.
• Statistical analysis: none.
• Administrative, technical, or material support: Aaron Yao.
• Study supervision: Aaron Yao, Dae Hyun Kim.
Corresponding author
Ethics declarations
Ethical Approval:
This is a non-human subjects research.
Conflict of Interest:
None.
Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior Presentations: None.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, A., Gao, L., Zhang, J. et al. Frailty as an Effect Modifier in Randomized Controlled Trials: A Systematic Review. J GEN INTERN MED 39, 1452–1473 (2024). https://doi.org/10.1007/s11606-024-08732-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-024-08732-8