Abstract
Background
Collaborative care (CC) is a multicomponent team-based approach to providing mental health care with systematic integration into outpatient medical settings. The 12-month INDEPENDENT CC intervention improved joint disease control measures in patients with both depression and diabetes at 12 and 24 months following randomization.
Objective
This study investigated the durability of intervention effects on patient outcomes at 36 months following randomization.
Participants
Adult patients with poorly controlled T2D and depression in India randomized to CC or usual care.
Design
Post hoc analyses of between-group differences in patient outcomes at 36 months post-randomization (N = 331) and maintenance of outcomes from 12 to 36 months (N = 314).
Main Measures
We evaluated combined risk factor improvement since baseline, defined as ≥ 50.0% reduction in Symptom Checklist Depression Scale (SCL-20) scores along with reduction of at least 0.5 percentage point hemoglobin A1C, 5 mmHg systolic blood pressure, or 10 mg/dL low-density lipoprotein cholesterol. Improvements in single risk factors were also examined.
Key Results
There were no between-group differences in improvements since baseline in multiple or single risk factors at 36 months. Patients in the CC group with improved outcomes at 12 months were more likely to maintain a ≥ 50.0% reduction since baseline in SCL-20 scores (CC [54.9%] vs. UC [40.9%]; RR: 1.27 [95% CI: 1.04, 1.56]) and 0.5 percentage point reduction since baseline in hemoglobin A1C (CC [31.9%] vs. UC [19.5%]; RR: 1.64 [95% CI: 1.11, 2.41]) at 36 months.
Conclusions
While improvements since baseline in patient outcomes did not differ between the collaborative care and usual care groups at 36 months, patients who received CC were more likely to maintain improvements in depressive symptoms and glucose levels at 36 months if they had achieved these improvements at the end of active intervention.
Trial Registration Number
Similar content being viewed by others
BACKGROUND
Up to one-third of adults with diabetes experience depression.1–4 Depression among patients with type 2 diabetes is associated with decreased adoption of recommended diabetes self-management behaviors, such as dietary modification, physical activity, medication adherence, and/or blood glucose monitoring.5–7 Among people with type 2 diabetes, depression is associated with increased risk of microvascular and macrovascular complications, including death.8, 9 Effective management of diabetes in people with co-morbid depression therefore requires addressing mental health alongside cardiometabolic risk factors.
The prevalence of diabetes is rising in many settings which also often experience a critical shortage of mental health specialists (e.g., psychiatrists), leading to a need for effective and cost-effective interventions for people with chronic physical and mental health conditions.10–12 The World Health Organization (WHO) has supported collaborative care as an effective and feasible strategy to increase access to mental health services for people who are also being treated for other chronic conditions.13 The Integrating Depression and Diabetes Treatment (INDEPENDENT) trial investigated and reported the beneficial effects of a 12-month collaborative care model vs. usual care to treat and manage type 2 diabetes and co-morbid depression in diabetes clinics in India.14 A higher proportion of participants in the intervention group achieved composite reductions in diabetes and depression care targets at 12 months and 24 months after randomization.14 In this analysis, we assessed the effects of this collaborative care model on cardiometabolic outcomes at 36 months after randomization.
DESIGN AND MEASURES
Study Design
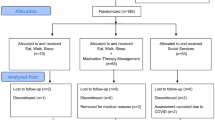
INDEPENDENT was a parallel, open-label, pragmatic randomized control trial (RCT). Details regarding the rationale, recruitment, randomization, and intervention have been published.14, 15 Briefly, patients 35 years or older with type 2 diabetes, moderate-to-severe depression (PHQ-9 score ≥ 10), and one or more uncontrolled cardiometabolic risk factor were recruited from four (private and public) diabetes clinics in India between 2015 and 2016 (Appendix Fig. 3). Patients (N=404) were randomized to either collaborative care or usual care for a 12-month active intervention period. Participants in the intervention group received the collaborative treatment model for 12 months. The trial evaluated outcomes at 12 months (the end of active intervention) and at 24 months (the end of 12 months of post-intervention passive follow-up) following randomization. The present study reports outcomes on participants who were followed 36 months following randomization (i.e., 24 months following active intervention). Institutional ethics committees at each participating site and the coordinating centers (i.e., Madras Diabetes Research Foundation and Emory University) approved the study, and eligible patients gave written informed consent prior to enrollment.
Intervention and Control Conditions
The intervention was collaborative care. Collaborative care is a multicomponent team-based approach to providing mental health care with systematic integration into outpatient medical settings, with an interdisciplinary team comprised of a primary medical provider, a mental health care manager, and a consulting psychiatrist collaborating to systematically track patient progress and deliver evidence-based care, including pharmacotherapy and/or brief behavioral interventions.16 Over 12 months, the collaborative care group received (1) notification to the diabetes care provider of depression status; (2) patient support and follow-up by care coordinators trained in nutrition and diabetes management and basic training in behavioral activation and motivational interviewing before the beginning of the trial; (3) clinical decision support advisories on medical management of glucose, blood pressure, lipids, and depression; and (4) bi-monthly collaborative case review meetings among the care coordinator, a diabetologist, and a psychiatrist to review the treatment plans and response to treatment of all patients in active treatment with the team intensify treatment for those who were not improving. The control group received usual care with provider notification of patient depression status during the 12-month intervention period.
Assessments
Patients in both arms completed in-person research assessments by trained and blinded study staff at baseline, every 6 months thereafter for 24 months, and at 36 months following randomization from March 2015 to November 2019. Baseline assessment included detailed demographic background, health history, and clinical and laboratory metrics. Thereafter, clinical and laboratory metrics and adverse events were collected at all follow-up visits. Outcome assessors were trained study staff employed at each site. They underwent training in study procedures and standardized outcome assessment alongside clinicians and care coordinators over a 3-day in-person training at the Madras Diabetes Research Foundation, the trial coordinating center.
Objectives
Outcomes
The collaborative care intervention targeted improvements in depressive symptoms (measured by the Symptom Checklist Depression Scale), glycemic control (measured by glycosylated hemoglobin (HbA1c)), blood pressure control (measured by systolic blood pressure (SBP)), and lipid control (measured by low-density lipoprotein cholesterol (LDL-c)). The primary outcome of interest was multiple risk factor improvement, defined as the percentage of patients who had at least 50% improvement in SCL-20 scores and a reduction of either a ≥ 0.5 percentage point in HbA1c, ≥ 5 mm Hg in SBP, or ≥ 10 mg/dL in LDL cholesterol since baseline. In addition, we examined levels and improvements since baseline in single risk factors and attainment of diabetes care goals (i.e., HbA1c <7% [53 mmol/mol], SBP <130 mm Hg, and LDL-C <100 mg/dL without history of CVD or LDL-C <70 with history of CVD). Finally, we examined outcome maintenance, defined as achieving the outcome at both the 12-month and 36-month time points.
Statistical Analysis
We described participant background characteristics at baseline, 12 months, and 36 months, and study outcomes at baseline, 12 months, 24 months, and 36 months, by treatment assignment.
Following intention-to-treat principles, we conducted a post hoc analysis of INDEPENDENT participants retained in follow-up at 36 months. We estimated adjusted risk ratios (RRs) of between-group differences in improvements in multiple and single risk factors from baseline to 36 months using log-binomial regression or Poisson regression in models that did not converge. Between-group differences of change in mean levels of outcomes from baseline to 36 months were estimated using ordinary least-squares regression. All models adjusted for study site. Uncertainty in intervention effects was assessed with 95% confidence intervals.
In addition, we examined between-group differences in the proportion of participants that maintained improvements in multiple and single risk factors from 12 months (end of active intervention) to 36 months (extended follow-up). This was an intention-to-treat analysis with all individuals randomized and assessed at 36 months in the denominator. In the numerator, we included individuals who had achieved an outcome at 12 months and maintained that same outcome at 36 months. This analysis was conducted to examine whether individuals who benefited from the intervention at 12 months maintained those benefits at 36 months.
To provide context for outcomes at 36 months, we also report the between-group differences observed at the end of the 12-month active intervention period. Potential heterogeneity of intervention effects at 36 months by age, sex, education, household income, duration of diabetes, and study site was evaluated through interaction terms between these factors and the intervention assignment.
Finally, we examined between-group differences in a composite measure of all four outcomes simultaneously, or a common effect. The common effect was the sum of standardized continuously measured SCL-20 scores, HbA1c, SBP, and LDL centered at a mean of zero with standard deviation of one (i.e., the standard normal distribution). The normalization allows comparison of outcomes on the same scale to be compared.
To evaluate differential retention of study participants, we compared the baseline characteristics of participants who were retained in follow-up at 36 months after randomization to those who were lost to follow-up overall and by intervention group (see Appendix Fig. 4 for participant flow). When compared to those who were lost to follow-up, participants who were assessed at 36 months were more likely to have a lower baseline SCL-20 score, lower A1C, lower FBG, and higher income (Appendix Table 3). Participants might have been lost to follow-up because of migration, cessation of interest to participate, disability, or death. To account for potential selection bias due to differential retention, we applied inverse probability weighting (IPW) to all analyses regarding trial effects. Inverse probability weights were applied to create a weighted analytic sample that resembled the full study population as randomized with respect to baseline characteristics.17
Statistical analysis and data visualizations were conducted using SAS version 9.4 and R version 4.1.3. We followed CONSORT guidelines in reporting results.18
RESULTS
Among 404 patients randomized at baseline, mean age was 53 years, and 59% were female. Baseline demographic and clinical characteristics of the collaborative care and usual care groups were balanced at randomization (Table 1). Similarly, baseline characteristics among participants observed (N = 331) compared with participants not observed (N=73) at 36 months were largely the same (Appendix Table 3). From baseline to 36 months, participants were on average 0.2 years older (52.7 years old vs. 52.9 years old) and heavier (BMI 27 kg/m2 vs. 29 kg/m2). No other demographic or clinical background characteristics changed during the study period.
From baseline to 36 months, SCL-20 scores improved (intervention: 1.3 to 0.4; control: 1.3 to 0.4), HbA1c improved (intervention: 1.3 to 0.4; control: 1.3 to 0.4), SBP stayed the same (intervention: 1.3 to 0.4; control: 1.3 to 0.4), and LDL increased (intervention: 1.3 to 0.4; control: 1.3 to 0.4) (Fig. 1). The SCL-20 scores in both the collaborative care and usual care groups progressively declined with each follow-up since randomization. In contrast, following randomization, there were initial reductions followed by increases in mean HbA1c, SBP, and LDL levels in both groups (Fig. 1). As a result, unadjusted between-group differences in improvements in SCL-20 and HbA1c were observed at 12 and 24 months but were not maintained at 36 months (Fig. 2).
In models accounting for study site and differential follow-up (N = 331), there was no intervention effect on the composite outcome at the end of the 36-month extended follow-up (Table 2; Appendix Table 4): 57.0% of the collaborative care group vs. 60.5% of the usual care group met the criteria for multiple risk factor control (RR: 0.93; 95% CI: 0.80, 1.09). Improvements in multiple risk factors at 36 months also did not vary by age, sex, education level, household income, duration of type 2 diabetes, or intervention site (Appendix Table 5). Furthermore, improvements since baseline in single risk factors, single risk factor targets, and diabetes care goals did not differ between collaborative care and usual care groups at the end of 36 months.
Our analysis of maintenance of intervention effects—or maintaining the study outcome from the end of active intervention (12 months) to extended follow-up (36 months)—considered data from 314 participants assessed at both 12 months and 36 months. We did not find between-group differences for improvements in combined depressive symptoms and cardiometabolic risk factors (38.3% vs. 30.7%, respectively; RR: 1.22 [95% CI: 0.92, 1.62]) (Table 2). Additionally, in the collaborative care group, 62 out of 104 (59%) patients had achieved combined improvements in cardiometabolic and depressive symptoms at 12 and 36 months; in the usual care group, 11 out of 68 (16%) had achieved cardiometabolic and depressive symptom improvements at 12 and 36 months. However, patients in the collaborative care group were more likely than the usual care group to maintain a ≥ 50% improvement in SCL-20 score (54.9% vs. 40.9%, respectively; RR: 1.27 [95% CI: 1.04, 1.56]) and a ≥ 0.5 percentage point reduction in HbA1c (31.9% vs. 19.5%, respectively; RR: 1.64 [95% CI: 1.11, 2.41]) (Table 2). No other statistically significant differences were observed in the maintenance of improvements in single risk factors or diabetes care targets.
We also found there was no statistically significant effect of the collaborative care model on the sum of standardized SCL-20 score, HbA1c, SBP, and LDL at either 12 months (−0.3 [95% CI: −0.33, −0.12]) or 36 months (0.04 [95% CI: −0.09 to 0.17]) (Appendix Table 6).
DISCUSSION
We examined the long-term effects of a 12-month collaborative care intervention for patients with poorly controlled diabetes and depressive symptoms attending four (private or public) urban clinics in India. Three years after randomization, or 2 years after the end of active intervention, there were no differences in clinically meaningful reductions of combined cardiometabolic indicators and depressive symptoms between the collaborative care and usual care groups. Patients in the collaborative care group, however, were more likely to exhibit reductions since baseline in HbA1c and depressive symptoms at both 12 and 36 months. There were no effects of collaborative care on any measure of elevated blood cholesterol or blood pressure at 36 months.
Our analyses indicate modest but promising effects of collaborative care on 36-month outcomes only for patients who experienced improvements at the end of active intervention. The collaborative care group was 1.27 and 1.64 times more likely to maintain reductions since baseline in depressive symptoms and HbA1c, respectively, at both 12 and 36 months. While the absolute percentage of patients in collaborative care who maintained target reductions in depressive symptom (55%) and HbA1c (31%) at both 12 and 36 months was modest, such improvements have implications for even longer-term maintenance of these metabolic goals.19, 20 Using process evaluations to identify which patient groups had longer-lasting benefits versus those who did not will be important in understanding how to improve collaborative care for depression and type 2 diabetes care going forward.21
The lack of between-group differences in multiple and single risk factor control at the 36-month follow-up may be explained by reductions in mean depression scores and increases in metabolic measures in both groups after the end of the 12-month active intervention period. At 12 months, between-group differences in multiple risk factor improvement were largely driven by improvements since baseline in glycemic control and depressive symptoms; there was no effect of the intervention on reductions in blood pressure or cholesterol at 24 months.14 By 24 months, between-group differences in glycemic control and depressive symptoms were substantially diminished, and by 36 months, no intervention effect was apparent on any study outcome.
The common improvements in depressive scores in both groups may be a product of genuine intervention effects or the natural progression of the course of depression.22 With respect to intervention effects in the usual care group, the provider was notified of even usual care patients were notified of depression status in clinics where non-regular screening for depression was occurring. This component of notifying the provider of the patient’s depression status might have prompted more action from the provider and thereby an increased perception of visibility and legitimacy of diagnosis from the patient. This may be one explanation for why both groups experienced reduced depression symptoms at 36 months. In addition, depression is episodic for many individuals. The high degree of symptom reduction in even the usual care group may be related to its natural history in the population. Others have suggested social desirability bias, regression to the mean, and a combination of the factors described here as reasons for reductions in depressive symptoms in control groups.23
It is notable that at 36 months, ~60% of all trial participants achieved improvements since baseline in multiple risk factors. Based on analysis of single risk factors, it appears that improvements in depressive symptoms were the driving force behind this outcome. Roughly three-quarters of patients achieved at least a 50% improvement since baseline in SCL-20 score at 36 months. In contrast, improvements since baseline in HbA1c were observed in only 44% of all trial participants, and the mean HbA1c level was 9%, at 36 months. These findings suggest that in this patient population glycemic improvements and glycemic control may rebound after active intervention. The regression of cardiometabolic indicators to pre-intervention levels has been reported in long-term follow-up studies of comparable collaborative care interventions.24–26
A novel strength of this study includes our evaluation of patient outcomes 3 years after randomization. Few studies follow patients, even more notably patients in LMICs and/or receiving a pragmatic intervention, for this extended duration. An additional strength of this study includes the intention-to-treat analysis building on a randomized controlled design. Through this design, we were able to investigate the effect of collaborative care on an array of standardized outcomes in an understudied but high-burden population.
This study also has limitations. This was a post hoc analysis of long-term outcomes, which the parent trial was not designed to study. As a result, we may not have had sufficient sample size to detect effects at 36 months. For example, the relative risk of maintaining the primary outcome and maintaining objective glycemic control at 36 months favored the intervention, but was not statistically significant, possibly due to insufficient sample size. While we were able to analyze data on over 80% of the participants randomized, there were some imbalances in patient characteristics by treatment group at 36 months. We addressed this limitation through IPW to address selection bias. Nevertheless, we cannot eliminate selection bias due to unobserved characteristics that differentially affected attrition.
CONCLUSIONS
Improvements since baseline in outcomes in patients with co-morbid type 2 diabetes and depression in India did not differ between the collaborative care and usual care groups at 36 months, largely due to a reduction in depressive symptoms and a rise in HbA1c in both treatment groups. There was evidence of intervention effects on maintaining improvements since baseline in depressive symptoms and glucose levels at both 12 and 36 months, suggesting that there were enduring outcome benefits for patients if they had achieved these improvements at the end of active intervention. Process evaluations to identify which patients benefit the most at the end of active intervention, and which patients tend to maintain those benefits, are priority research areas to calibrate collaborative care for depression and type 2 diabetes care for this population.
References
Garin N, Koyanagi A, Chatterji S, et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. J Gerontol A Biol Sci Med Sci. 2016;71(2):205-214. https://doi.org/10.1093/gerona/glv128.
Sagar R, Dandona R, Gururaj G, et al. The burden of mental disorders across the states of India: the global burden of disease study 1990–2017. Lancet Psychiatry. 2020;7(2):148-161. https://doi.org/10.1016/S2215-0366(19)30475-4.
Patel V, Chatterji S. Integrating mental health in care for noncommunicable diseases: an imperative for person-centered care. Health Affairs. 2015;34(9):1498-1505. https://doi.org/10.1377/hlthaff.2015.0791.
van Oostrom SH, Picavet HSJ, van Gelder BM, et al. Multimorbidity and comorbidity in the Dutch population – data from general practices. BMC Public Health. 2012;12(1):715. https://doi.org/10.1186/1471-2458-12-715.
Alzoubi A, Abunaser R, Khassawneh A, Alfaqih M, Khasawneh A, Abdo N. The bidirectional relationship between diabetes and depression: a literature review. Korean J Fam Med. 2018;39(3):137-146. https://doi.org/10.4082/kjfm.2018.39.3.137.
CDC. Diabetes and Mental Health. Centers for Disease Control and Prevention. Published August 6, 2018. https://www.cdc.gov/diabetes/managing/mental-health.html. Accessed 27 Apr 2021.
Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851-858. https://doi.org/10.1016/S0140-6736(07)61415-9.
Dooren FEP van, Nefs G, Schram MT, Verhey FRJ, Denollet J, Pouwer F. Depression and risk of mortality in people with diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. 2013;8(3):e57058. https://doi.org/10.1371/journal.pone.0057058.
Nouwen A, Adriaanse MC, Dam K van, et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med. 2019;36(12):1562-1572. https://doi.org/10.1111/dme.14054.
Basu S, Sharma N. Diabetes self-care in primary health facilities in India - challenges and the way forward. World J Diabetes. 2019;10(6):341-349. https://doi.org/10.4239/wjd.v10.i6.341.
Garg K, Kumar CN, Chandra PS. Number of psychiatrists in India: baby steps forward, but a long way to go. Indian J Psychiatry. 2019;61(1):104-105. https://doi.org/10.4103/psychiatry.IndianJPsychiatry_7_18.
Kasthuri A. Challenges to healthcare in India - the Five A’s. Indian J Community Med. 2018;43(3):141-143. https://doi.org/10.4103/ijcm.IJCM_194_18.
WHO. Integrating the response to mental health disorders and other chronic diseases in health care systems. WHO. https://www.who.int/mental_health/publications/gulbenkian_paper_integrating_mental_disorders/en/. Accessed 27 Apr 2021.
Ali MK, Chwastiak L, Poongothai S, et al. Effect of a collaborative care model on depressive symptoms and glycated hemoglobin, blood pressure, and serum cholesterol among patients with depression and diabetes in India: the INDEPENDENT Randomized Clinical Trial. JAMA. 2020;324(7):651-662. https://doi.org/10.1001/jama.2020.11747.
Kowalski AJ, Poongothai S, Chwastiak L, et al. The INtegrating DEPrEssioN and Diabetes treatmENT (INDEPENDENT) Study: design and methods to address mental healthcare gaps in India. Contemp Clin Trials. 2017;60:113-124. https://doi.org/10.1016/j.cct.2017.06.013.
Katon W. Collaborative depression care models: from development to dissemination. Am J Prev Med 2012;42(5):550–552. https://doi.org/10.1016/j.amepre.2012.01.017.
Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60(7):578-586. https://doi.org/10.1136/jech.2004.029496.
Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med 2010;152.
Hamdy O, Mottalib A, Morsi A, El-Sayed N, Goebel-Fabbri A, Arathuzik G, Shahar J, Kirpitch A, Zrebiec J. Long-Term Effect of Intensive Lifestyle Intervention on Cardiovascular Risk Factors in Patients with Diabetes in Real-World Clinical Practice: a 5-Year Longitudinal Study. BMJ Open Diabetes Res Care 2017;5(1): e000259. https://doi.org/10.1136/bmjdrc-2016-000259.
Jancey J, Lee ah, James AP, Howat P, Hills AP, Anderson AS, Tran VD, Blackford K. Long-term sustainability of a physical activity and nutrition intervention for rural adults with or at risk of metabolic syndrome. Aust N Z J Public Health 2020;44(5): 421–26. https://doi.org/10.1111/1753-6405.13036.
Johnson LCM, Chwastiak L, Poongothai S, Tandon N, Anjana RM, Aravind S, Sridhar GR, Rao D, Mohan V, Ali MK. Adaptations and Patient Responses to Behavioral Intervention Components in a Depression-Focused Chronic Disease Care Model Implemented in India. Transl Behav Med 2020;10(1): 35–45. https://doi.org/10.1093/tbm/ibz192.
Beshai S, Dobson KS, Bockting CLH, Quigley L. Relapse and Recurrence Prevention in Depression: Current Research and Future Prospects. Clin Psychol Rev 2011;31(8): 1349–60. https://doi.org/10.1016/j.cpr.2011.09.003.
Grimm P. Social Desirability Bias. In: Wiley International Encyclopedia of Marketing. American Cancer Society; 2010. https://doi.org/10.1002/9781444316568.wiem02057.
Katon WJ, Von Korff M, Lin EHB, et al. The pathways study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61(10):1042. https://doi.org/10.1001/archpsyc.61.10.1042.
McAdam-Marx C, Dahal A, Jennings B, Singhal M, Gunning K. The effect of a diabetes collaborative care management program on clinical and economic outcomes in patients with type 2 diabetes. J Manag Care Spec Pharm. 2015;21(6):452-468. https://doi.org/10.18553/jmcp.2015.21.6.452.
Sandbæk A, Griffin SJ, Sharp SJ, et al. Effect of early multifactorial therapy compared with routine care on microvascular outcomes at 5 years in people with screen-detected diabetes: a randomized controlled trial: the ADDITION-Europe Study. Diabetes Care. 2014;37(7):2015-2023. https://doi.org/10.2337/dc13-1544.
Acknowledgements
Contributors
We thank the entire INDEPENDENT study team and INDEPENDENT study participants for their contributions and time to this study. We also thank Halley Riley, MPH, and Rob O’Reilly, PhD, from the Emory Center for Digital Scholarship, for inputs on statistical methodology.
Funding
The INDEPENDENT trial was funded by the National Institute of Mental Health (grant R01MH100390). This study did not receive any additional funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No authors reported a conflict of interest with this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 204 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suvada, K., Ali, M.K., Chwastiak, L. et al. Long-term Effects of a Collaborative Care Model on Metabolic Outcomes and Depressive Symptoms: 36-Month Outcomes from the INDEPENDENT Intervention. J GEN INTERN MED 38, 1623–1630 (2023). https://doi.org/10.1007/s11606-022-07958-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-022-07958-8