Abstract
Background
The incidence of diabetes in the general US population (6.7 per 1000 adults in 2018) has not changed significantly since 2000, suggesting that individuals with prediabetes are not connecting to evidence-based interventions.
Objective
We sought to describe the clinical care of individuals with prediabetes, determine patient factors associated with this care, and evaluate risk for diabetes development.
Design
Retrospective cohort study using linked claims and electronic health record data.
Participants
We created a cohort of adults with prediabetes based on laboratory measures. We excluded patients with a prior history of diabetes, pregnancy in prior 6 months, or recent steroid use.
Main Measures
We measured ordering and completion of clinical services targeting prediabetes management and diabetes incidence within 12 months following cohort entry. We tested the strength of the association between individuals’ characteristics and outcomes of interest using bivariate and multiple logistic regression.
Results
Our cohort included 3888 patients with a laboratory diagnosis of prediabetes (incident or prevalent prediabetes). Within 12 months, 63.4% had repeat glycemic testing, yet only 10.4% had coded diagnoses of prediabetes, 1.0% were referred for nutrition services, and 5.4% were prescribed metformin. Most patients completed labs and nutrition visits when referred and filled metformin when prescribed. Individuals with a higher glycemic level or BMI were more likely to receive prediabetes clinical care. Six percent of individuals developed diabetes within 12 months of cohort entry and had higher glycemic levels and BMI ≥ 30 kg/m2. In the adjusted model, Black individuals had 1.4 times higher odds of developing diabetes than White individuals.
Conclusions
Rates of prediabetes clinical care activities are low and have not improved. Strategies are urgently needed to improve prediabetes care delivery thereby preventing or delaying incident diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Prediabetes affects 88 million US adults.1 Despite evidence-based treatments for diabetes prevention including Diabetes Prevention Programs (DPP), the incidence of diabetes has not decreased over time.1 Prior studies using electronic health record (EHR) data have demonstrated that primary care providers (PCPs) infrequently refer patients to nutritionists, assign a diagnosis of prediabetes, or prescribe metformin.2,3,4 Other studies have shown that PCPs rarely refer patients to DPPs5,6,7 and that enrollment and retention after referral is low.6 These findings suggest the lack of progress in reducing the incidence of diabetes may be partly explained by PCP’s treatment practices for prediabetes and patients’ lack of engagement in diabetes prevention efforts.
In 2021, the US Preventive Services Task Force (USPSTF) updated recommendations around screening for prediabetes and diabetes, highlighting important research gaps including identifying factors associated with risk of progression from prediabetes to diabetes.8 Therefore, within our large health system with multiple primary care sites and a diverse patient population, we sought to characterize the clinical care activities of PCPs for patients with prediabetes to identify gaps and risk factors for progression to inform an intervention to improve prediabetes care in the primary care clinic setting.
We conducted a retrospective cohort study using linked EHR and claims data. Our objectives were to (1) describe the prediabetes clinical care activities ordered by PCPs and their completion by patients, (2) determine what patient factors are associated with this care, and (3) understand the incidence of diabetes and whether PCP treatment practices and patient factors are associated with risk of progression to diabetes.
METHODS
Data
We used a linked claims and EHR dataset. Claims from patients with a Johns Hopkins Health Care insurance product (excluding Tricare) and EHR data from Johns Hopkins Medicine are linked in a database, which contains data on outpatient visits, referrals, outpatient prescription orders and fills, inpatient hospitalizations, and laboratory orders/results. We received a de-identified dataset that included any individual with EHR data plus their associated claims data in a 5-year window from February 2016 to February 2021.
Cohort
Using this linked dataset, we created a cohort of patients aged ≥ 18 years who had at least two outpatient primary care encounters and were covered under one of 3 insurance plans (Employee Health Program, Priority Partners Medicaid Managed Care Organization, and Medicare/Advantage MD) at any time during the study period. Due to a lack of insurance plan enrollment data, we required patients to have at least one claim in the 12 months prior to and in the 12 months after cohort entry. The cohort entry date was defined as the date with a laboratory measure indicating prediabetes [hemoglobin A1c (HbA1c) 5.7 to 6.4% or fasting glucose 100 to 125 mg/dL]. Since fasting status is not documented in our EHR, we included only glucose measurements or panels with glucose measurements drawn between 6 a.m. and 10 a.m. based on our prior study.9 We categorized patients as having incident prediabetes (normal lab followed by an abnormal lab in the prediabetes range) or prevalent prediabetes (abnormal lab in prediabetes range with no prior normal lab).
We excluded patients with any prior diagnosis of diabetes based on the SUPREME-DM definition,10 pregnancy in prior 6 months,11 or recent steroid use in prior 30 days (Fig. 1, Appendix 2). Each patient was attributed to a primary care clinic based on where their first primary care encounter occurred after cohort entry or their last encounter within 12 months prior to cohort entry if no primary care encounter occurred after cohort entry.
Characterizing the Cohort
We extracted demographic information (age, sex, race, insurer) at cohort entry from EHR data (Fig. 1). We used weight, body mass index (BMI), and blood pressure (BP) measurements collected at a PCP clinic encounter within 180 days before or 180 days after cohort entry. We assessed baseline conditions, specifically hypertension and chronic kidney disease, based on the Chronic Conditions Data Warehouse.12 We also assessed for history of gestational diabetes13 and calculated the Elixhauser comorbidity index14 using all available claims data before cohort entry. We defined baseline care utilization by summing the number of outpatient visits and inpatient hospitalizations that occurred in the 12 months prior to cohort entry and categorized it into quartiles.
Outcomes
Our primary and secondary outcomes were prediabetes clinical care activities and the factors associated with them. We separated prediabetes clinical care activities into PCP practices and patient behaviors. Using EHR data, we examined PCP visits, PCP visits coded with a diagnosis of prediabetes based on ICD-10 codes (Appendix 2), glycemic test orders and results, nutrition referral and visits, and metformin prescription. Additionally, we looked at nutrition visits using claims data to confirm findings from EHR data. We quantified metformin fills using claims data. Our tertiary outcome was development of diabetes based on the SUPREME-DM definition: ≥ 1 inpatient ICD code for diabetes or ≥ 2 of the following (outpatient ICD code for diabetes, labs in diabetes range, or diabetes medication fill)10 (Appendix 2). If a patient met diabetes criteria based on ≥ 1 inpatient ICD code, we took the date of this criterion as the index date of diabetes. If a patient met diabetes criteria based on ≥ 2 required criteria (outpatient code, labs, or medications), we took the later date of the two criteria as the index date of diabetes.
Statistical Analysis
For our primary objective, we used descriptive statistics to characterize the patients at cohort entry. We then tabulated the occurrence of each of the specific prediabetes clinical care activities within 3, 6, and 12 months following cohort entry (dichotomized as any or none). We tested the strength of the association between each baseline characteristic and each outcome of interest using bivariate logistic regression.
For our secondary objective, we used multiple logistic regression models to determine the strengths of the independent associations of our hypothesized predictors of prediabetes care and the outcomes of interest; these were age, sex, baseline BMI, race (Black, White, Asian, and other), and care utilization before cohort entry. We included clinic as a fixed effect in the model. We tested model fit using Laplace estimation.
For our tertiary objective, we used descriptive statistics to characterize the patients who developed diabetes within 3, 6, and 12 months following cohort entry. We tested the strengths of the association between baseline characteristics and the outcomes of interest using bivariate logistic regression. We evaluated independent predictors of diabetes using multiple logistic regression with clinic as a fixed effect. We tested model fit using Laplace estimation. This study was approved by the Johns Hopkins Institutional Review Board. All analyses were conducted using SAS 9.4.
RESULTS
Our cohort included 3888 patients with a laboratory diagnosis of prediabetes from 37 primary care clinics. At cohort entry, one-quarter of the patients had incident prediabetes and the rest had prevalent prediabetes. Mean age was 63 years (Table 1). The majority of patients were female (65.4%) and White (55.0%) or Black (34.5%); few were of Hispanic ethnicity (3.0%). Patients were most commonly insured by Medicare (7.1% managed care, 43.1% fee-for-service), commercial insurance (23.2%), and Medicaid (12.1% managed care, 0.5% fee-for-service). Mean BMI at baseline was 30.0 kg/m2 with over 40% of patients with BMI ≥ 30 kg/m2. Nearly half of the patients had a HbA1c of 6–6.4% and 15% had a glucose of 110–125 mg/dL.
Prediabetes Clinical Care Activities: PCP Practices
Among patients with at least one PCP visit after cohort entry, only 13.0% of these visits were coded with an ICD-10 diagnosis of prediabetes (Table 2). PCPs ordered follow-up glycemic testing for 63.4% of patients (28.1% metabolic panel only, 12.3% A1c only, and 59.5% with both tests). PCPs referred 1.0% of patients to a nutritionist and prescribed metformin for 5.4% of patients.
Prediabetes Clinical Care Activities: Patient Behaviors
Within 12 months of cohort entry, nearly 80% of patients had at least one PCP visit (Table 2). Although only half of the patients overall completed a glycemic test, of the 63.4% of patients who had an order, 79.2% completed it. Nutrition visits were uncommon with 1.0% of patients in the cohort having a visit within 12 months of diagnosis. Of the 1.0% of patients who were referred to nutrition, 75.7% attended a visit. Among the 5.4% of patients with a metformin prescription, 76.3% filled it.
Factors Associated with Prediabetes Clinical Care Activities
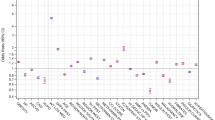
In bivariate analyses, patients with Asian race or BMI categories ≥ 25 kg/m2 were more likely to have prediabetes coded at a PCP visit compared to White race and BMI < 25 kg/m2, respectively (Table 3). Older age (age 45+ years), Medicare insurance, and higher baseline glycemic level were associated with higher rates of glycemic testing completion compared to age 18–26 years, commercial insurance, and lower baseline glycemic level, respectively. Metformin prescription was more common among Blacks, Asians, and other/multiracial patients; higher baseline glycemic level; and BMI ≥ 25 kg/m2 compared to Whites, lower baseline glycemic level, and BMI < 25 kg/m2, respectively. Nutrition visits were higher among Blacks and those with BMI ≥ 40 kg/m2 compared to Whites and BMI < 25 kg/m2, respectively.
In the multiple logistic regression model, independent predictors of a coded prediabetes diagnosis within 12 months included male sex, Asian race, and higher BMI (Appendix Tables S1 and S2). Independent predictors of glycemic lab test order and completion included older age. Less prior care utilization (quartile 1 vs. 4) and commercial insurance (vs. other insurance) were also significantly associated with glycemic lab test completion (Appendix Tables S1 and S2). For metformin prescription, independent predictors included older age, male sex, higher BMI, and less prior care utilization (quartile 1 vs. 2) (Appendix Tables S1 and S2). Asian race and Black race were associated with metformin prescription only among those with a lower baseline glycemic level. Independent predictors of metformin fill included higher BMI. Among patients with a higher baseline glycemic level, Medicare insurance was associated with lower odds of metformin prescription and fill. Asian race was a significant predictor of metformin fill in the lower baseline glycemic level group (Appendix Tables S1 and S2).
Incident Diabetes and Factors Associated with Development of Diabetes Within 12 Months
Six percent of patients with prediabetes (n = 249) developed incident diabetes within 12 months of cohort entry (3.5% of patients with incident prediabetes, 7.4% of patients with prevalent prediabetes). Patients who developed diabetes were more likely to have a higher glycemic level, BMI ≥ 30 kg/m2, and Black race at cohort entry compared to lower glycemic level, BMI < 25 kg/m2, and White race, respectively (Table 4). In the adjusted model, Black individuals were 1.4 times more likely to develop diabetes compared to White individuals (Table 5). Patients who developed diabetes were more likely to have completed a PCP visit, glycemic testing, or a nutrition visit in the 12 months after cohort entry compared to those who did not develop diabetes (Appendix Table S3).
DISCUSSION
Among patients with prediabetes receiving care in one large health system, we found that few patients had coded diagnoses of prediabetes, were referred to a nutritionist, or were prescribed metformin in the 12 months after cohort entry. In contrast, most patients completed labs and nutrition visits when referred and filled metformin when prescribed. Patients with higher glycemic levels or higher BMIs were more likely to receive these prediabetes clinical care activities although the proportion was low. These higher-risk patients were also more likely to develop diabetes within 12 months. Patients who developed diabetes were more likely to have had a PCP visit, completed glycemic testing, and/or attended a nutrition visit within 12 months after cohort entry, and we suspect the more frequent visits and testing provided more opportunity for patients to be diagnosed with diabetes. The higher rates of prediabetes clinical care activities may also reflect PCPs’ concern about their risk of progression to diabetes and therefore these activities were more likely to occur. The association between prediabetes clinical care activities and development of diabetes does not imply causality, and distinguishing the timing of these activities relative to the diabetes diagnosis is not straightforward as many of these activities occur both before and after diagnosis.
Our results are consistent with prior studies using claims and/or EHR data, 2,3,4 but add to current literature by examining risk factors for diabetes progression, a gap highlighted by the USPTF’s recent guidelines.8 In a Cleveland Clinic study using EHR data, among 16,713 patients with prediabetes, 5.4% received a metformin prescription and 5.7% received nutrition referrals during 1 year of follow-up.4 Higher BMI and higher HbA1c were both associated positively with treatment, similar to our findings. Although our rates of nutrition referrals were lower, these authors also found that Blacks were more likely to be referred to nutrition compared to Whites. Many patients in our cohort were insured by Medicare fee-for-service, which covers medical nutrition therapy only for people with diabetes and renal disease15 and may explain low rates of nutrition referral. Insurance was not described in the study of Cleveland Clinic patients. In an older Kaiser Permanente study using EHR data, the authors found that in the 6 months after diagnosis of prediabetes, 18% had repeat glycemic testing done and < 0.1% initiated metformin, rates much lower than ours.2
There are several limitations to note. Our data is from a single health system. The 5-year study period overlapped with the COVID-19 pandemic, and therefore, lab completion rates may have declined. However, telemedicine visits were included in our outcome of PCP visits. Glycemic testing included serum glucose drawn as part of a panel which may not have been ordered to check a fasting glucose and therefore may overestimate the prevalence of follow-up testing. We did not have insurance enrollment dates, and therefore, we would miss claims if patients switched insurance plans, away from a JHHC product, after cohort entry; for this reason, we set an inclusion criterion that patients must have at least one claim in the 12 months after cohort entry. We included both prevalent and incident prediabetes in our cohort, and baseline characteristics were similar in both groups (Appendix Table S4). Although we included clinic as a fixed effect to control for clinic differences, we did not examine other clinic-level predictors. Finally, a DPP at our institution exists; however, an EHR referral order was not implemented until 2020, and the program has had limited capacity to enroll patients. Therefore, we could not examine its use among our outcomes.
Compared to prior studies, strengths of our study include newer data (2016–2021) and use of both EHR and claims data to evaluate PCP practices and patient behaviors. Claims data allowed us to examine care delivered outside the health system. We looked at insurance type at baseline to see if prediabetes clinical care activities differed, which prior studies did not. We examined the development of diabetes which adds to the current limited data on risk of progression to diabetes among different racial/ethnic groups in the USA.16,17 Although prediabetes prevalence does not vary by race/ethnicity, national estimates show that the incidence of newly diagnosed diabetes is higher among Hispanics (9.0%), non-Hispanic Blacks (7.9%), and non-Hispanic Asians (7.2%) compared to non-Hispanic Whites (5.4%).1 National estimates also indicate that only 15.3% of adults with prediabetes report being told by a health professional that they had this condition with rates lowest among Hispanics (10.8%) and Asians (9.8%)1 suggesting that the underdiagnosis of prediabetes may help explain disproportionate rates of diabetes among racial/ethnic groups. In our study, we found that Black patients were 1.4 times more likely to develop diabetes compared to their White counterparts. In exploratory analyses (data not shown), we found that completion of glycemic testing and nutrition visits attenuated the relationship between race and the development of diabetes, suggesting the need for additional studies to understand if directing clinical services to higher-risk groups reduces the disproportionate rates of diabetes among races.
A number of studies have shown the benefits of clinical decision support tools in increasing DPP referrals.21,22,23 Future research is needed to understand whether other EHR clinical decision support tools can help improve prediabetes clinical care. PCP awareness of prediabetes screening is high,18 but knowledge about specific guideline recommendations is inadequate,19,20 and clinical decision support tools may help fill this gap. These tools could include streamlining orders (e.g., labs, metformin) and referrals (e.g., DPPs), and ensuring that the diagnosis is documented. A closed-loop referral system in the EHR is important for providing feedback to PCPs after a referral is placed to internal departments like nutrition or external community-based services like DPPs. Although we were unable to look at the outcome of DPP referrals, DPP enrollment and retention are a challenge highlighted in prior studies.24,25 Therefore, a closed-loop system with bi-directional communication between PCPs and clinical services may help to address possible barriers to patients attending nutrition appointments or DPP sessions which may not otherwise get communicated to PCPs. Finally, a prediabetes registry would allow a practice or health system to monitor the prevalence of prediabetes, uptake of evidence-based prediabetes clinical care activities, and impact of treatment on the development of diabetes among their patients with prediabetes.
Performance measures around prediabetes management may also improve practices around diagnosis, DPP and nutrition referrals, and metformin prescription. In our study, we found that few patients had a ICD-10 diagnosis of prediabetes coded, were referred to a nutrition visit, or were prescribed metformin in the 12 months following cohort entry, which are all quality measures for prediabetes proposed by the American Medical Association in 2019.26 Some studies among people with diabetes have demonstrated a positive effect of performance measurement on quality indicators and diabetes care.27
In conclusion, our study demonstrates that 6% of individuals with prediabetes develop diabetes within 1 year of follow-up. Despite this, rates of prediabetes clinical care activities remain low, suggesting little has changed in practices around diabetes prevention. We found that certain patient factors are associated with prediabetes clinical care activities and diabetes incidence. Strategies to improve prediabetes diagnosis, DPP and nutrition referrals, and metformin prescribing are urgently needed to improve prediabetes care delivery with the goal of preventing or delaying incident diabetes.
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA: Centers for Disease Control and Prevention; 2020.
Schmittdiel JA, Adams SR, Segal JB, et al. Novel use and utility of integrated electronic health records to assess rates of prediabetes recognition and treatment: brief report from an integrated electronic health records pilot study. Diabetes Care. 2014;37(2):565-8.
Moin T, Li J, Duru OK, et al. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med. 2015;162(8):542-8.
Speaker SL, Rastogi R, Sussman TA, Hu B, Misra-Hebert AD, Rothberg MB. Treatment of Patients with Prediabetes in a Primary Care Setting 2011-2018: an Observational Study. J Gen Intern Med. 2021;36(4):923-9.
Venkataramani M, Pollack CE, Yeh HC, Maruthur NM. Prevalence and Correlates of Diabetes Prevention Program Referral and Participation. Am J Prev Med. 2019;56(3):452-7.
Ali MK, McKeever Bullard K, Imperatore G, et al. Reach and Use of Diabetes Prevention Services in the United States, 2016-2017. JAMA Netw Open. 2019;2(5):e193160.
Brunisholz KD, Conroy MB, Belnap T, Joy EA, Srivastava R. Measuring Adherence to U.S. Preventive Services Task Force Diabetes Prevention Guidelines Within Two Healthcare Systems. J Healthc Qual. 2021;43(2):119-25.
Davidson KW, Barry MJ, Mangione CM, et al. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(8):736-43.
Tseng E, Segal JB, Maruthur NM. Fasting Status of Patients Undergoing Ambulatory Laboratory Testing. Diabetes Care. 2019;42(8):e133-e4.
Raebel MA SE, Goodrich G, Paolino AR, et al. Validating type 1 and type 2 diabetes mellitus in the Mini-Sentinel Distributed Database using the Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM) DataLink. 2016.
MacDonald SC, Cohen JM, Panchaud A, McElrath TF, Huybrechts KF, Hernández-Díaz S. Identifying pregnancies in insurance claims data: Methods and application to retinoid teratogenic surveillance. Pharmacoepidemiol Drug Saf. 2019;28(9):1211-21.
Chronic Conditions Data Warehouse [Internet]. Centers for Medicare & Medicaid Services; [cited 2020 Aug 1]. Available from: https://www2.ccwdata.org/web/guest/condition-categories.
Herrick CJ, Keller MR, Trolard AM, Cooper BP, Olsen MA, Colditz GA. Factors Associated With Postpartum Diabetes Screening in Women With Gestational Diabetes and Medicaid During Pregnancy. Am J Prev Med. 2021;60(2):222-31.
Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-33.
Medical Nutrition Therapy Benefit for Diabetes & ESRD [Internet]. Baltimore, MD: Centers for Medicare & Medicaid Services; [cited 2021 Sept 1]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId = 53&fromdb = true.
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New Engl J Med. 2002;346(6):393-403.
Tabak AG, Herder C, Rathmann W. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279-90.
Nhim K, Khan T, Gruss SM, et al. Primary Care Providers' Prediabetes Screening, Testing, and Referral Behaviors. Am J Prev Med. 2018;55(2)e39-e47.
Tseng E, Greer RC, O'Rourke P, et al. Survey of primary care providers' knowledge of screening for, diagnosing and managing prediabetes. J Gen Intern Med. 2017;32(11):1172-8.
Tseng E, Greer RC, O'Rourke P, et al. National Survey of Primary Care Physicians' Knowledge, Practices, and Perceptions of Prediabetes. J Gen Intern Med. 2019;34(11):2475-81.
Chambers EC, Wylie-Rosett J, Blank AE, et al. Increasing Referrals to a YMCA-Based Diabetes Prevention Program: Effects of Electronic Referral System Modification and Provider Education in Federally Qualified Health Centers. Prev Chronic Dis. 2015;12:E189.
Holliday CS, Williams J, Salcedo V, Kandula NR. Clinical Identification and Referral of Adults With Prediabetes to a Diabetes Prevention Program. Prev Chronic Dis. 2019;16:E82.
Nhim K, Khan T, Gruss S, et al. Facilitators to referrals to CDC's National Diabetes Prevention Program in primary care practices and pharmacies: DocStyles 2016-2017. Prev Med. 2021;149:106614.
Cannon MJ, Masalovich S, Ng BP, et al. Retention Among Participants in the National Diabetes Prevention Program Lifestyle Change Program, 2012–2017. Diabetes Care. 2020;43(9):2042-9.
Howarth E, Bower PJ, Kontopantelis E, et al. 'Going the distance': an independent cohort study of engagement and dropout among the first 100 000 referrals into a large-scale diabetes prevention program. BMJ Open Diabetes Res Care. 2020;8(2):e001835.
Prediabetes Quality Measures [Internet]. American Medical Association; [cited 2021 Sept 1]. Available from: https://amapreventdiabetes.org/sites/default/files/uploaded-files/AMA%20Prediabetes%20Measures_REVISED%20FINAL_20DEC19.pdf.
O'Connor PJ, Bodkin NL, Fradkin J, et al. Diabetes Performance Measures: Current Status and Future Directions. Diabetes Care. 2011;34(7):1651-9.
Funding
Dr. Eva Tseng is supported by the National Institute of Diabetes and Digestive and Kidney Diseases [K23DK118205].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tseng, E., Durkin, N., Clark, J.M. et al. Clinical Care Among Individuals with Prediabetes in Primary Care: a Retrospective Cohort Study. J GEN INTERN MED 37, 4112–4119 (2022). https://doi.org/10.1007/s11606-022-07412-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-022-07412-9