Abstract
Background
Sedative-hypnotics are frequently prescribed for insomnia in hospital but are associated with preventable harms.
Objective, Design, and Participants
We aimed to examine whether a sedative-hypnotic reduction quality improvement bundle decreases the rate of sedative-hypnotic use among hospitalized patients, who were previously naïve to sedative-hypnotics. This interrupted time series study occurred between May 2016 and January 2019. Control data for 1 year prior to implementation and intervention data for at least 16 months were collected. The study occurred on 7 inpatient wards (general medicine, cardiology, nephrology, general surgery, and cardiovascular surgery wards) across 5 teaching hospitals in Toronto, Canada.
Intervention
Participating wards implemented a sedative-hypnotic reduction bundle (i.e., order set changes, audit-feedback, pharmacist-enabled medication reviews, sleep hygiene, daily sleep huddles, and staff/patient/family education) aimed to reduce in-hospital sedative-hypnotic initiation for insomnia in patients who were previously naïve to sedative-hypnotics. Each inpatient ward adapted the bundle prior to sustaining the intervention for a minimum of 16 months.
Main Measures
The primary outcome measure was the proportion of sedative-hypnotic-naïve inpatients newly prescribed a sedative-hypnotic for sleep in hospital. Secondary measures include prescribing rates of other sedating medications, fall rates, length of stay, and mortality.
Key Results
We included 8,970 patient discharges in the control period and 10,120 in the intervention period. Adjusted sedative-hypnotic prescriptions among naïve patients decreased from 15.48% (95% CI: 6.09–19.42) to 9.08% (p<0.001) (adjusted OR 0.814; 95% CI: 0.667–0.993, p=0.042). Unchanged secondary outcomes included mortality (adjusted OR 1.089; 95% CI: 0.786–1.508, p=0.608), falls (adjusted rate ratio 0.819; 95% CI: 0.625–1.073, p=0.148), or other sedating drug prescriptions (adjusted OR 1.046; 95% CI: 0.873–1.252, p=0.627).
Conclusions
A sedative-hypnotic reduction quality improvement bundle implemented across 5 hospitals was associated with a sustained reduction in sedative-hypnotic prescriptions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
BACKGROUND
Sedative-hypnotic medications are commonly prescribed in hospital for insomnia despite the absence of high-quality evidence that they are even effective sleep aids.1In addition, in-hospital initiation of sedative-hypnotics is associated with on-going prescription use following discharge despite recommendations against chronic use for insomnia.1,2,3,4Sedative-hypnotic use remains prevalent in spite of well-documented harms such as falls.5,6,7 As a result, professional societies via the Choosing Wisely Campaign advocate for de-adoption of this non-evidence-based, and potentially harmful prescribing practice.8,9
Reducing sedative-hypnotic initiation among those who are previously naïve to these medications requires a focus on its unique drivers and potential solutions addressing root causes.3,4 Effective methods to reduce sedative-hypnotic prescribing include interventions such as creating a sleep-friendly environment and prescriber education, medication reviews by pharmacists, or clinical decision support.10 Previously, we described a successful single-center intervention associated with reduced naïve inpatient sedative-hypnotic prescriptions.11 Using quality improvement methods, we developed an intervention bundle (admission order set changes, education to staff/patients/families, and implementation of sleep hygiene practices) based on the contributors (“routine” sedative orders to preemptively address insomnia on admission, lack of awareness of harms of sedative-hypnotics, and disruptive sleep environment in hospitals) to naïve inpatient sedative prescribing. In the current study, we aimed to implement a sedative-hypnotic reduction bundle across multiple inpatient settings and evaluate its impact on reducing sedative-hypnotic-naïve patient exposure to potential harms.
METHODS
Context
Based on a prior study, one of the participating centers reported high and potentially inappropriate rates of sedative-hypnotic use for insomnia.4Sedative-hypnotics of interest included all benzodiazepines and nonbenzodiazepine γ-aminobutyric acid receptor agonists available at all sites during the study period, zopiclone and zolpidem. Inpatient wards were selected based on availability of physician leads with capacity to conduct this project and lack of concurrent ward initiatives that would impact the outcome. Hospital 1 is a 442-bed academic medical center, hospital 2 is a 471-bed academic medical center, hospital 3 is a 256-bed academic medical center, hospital 4 is a 455-bed academic medical and trauma center, and hospital 5 is a 627-bed academic medical and trauma center. A total of 7 inpatient wards participated in the study with the following distribution: hospital 1: general surgery (1 ward); hospital 2: general medicine (2 wards); hospital 3: cardiology (1 ward); hospital 4: general medicine (1 ward); hospital 5: nephrology (1 ward) and cardiovascular surgery (1 ward). Four hospitals have computerized provider order entry (CPOE) systems and all are located in a large urban setting in Toronto, Canada.
Study Design
This observational multi-centered study using an interrupted time series design aimed to reduce potentially inappropriate sedative-hypnotic prescribing among naïve inpatients across five teaching hospitals. Participating quality improvement (QI) teams were permitted to modify the previously studied sedative-hypnotic reduction bundle using QI methods (such as plan-do-study-act(PDSA) cycles) to adapt the intervention to meet local needs while preserving core aspects of the intervention.12 Local improvement teams entered the intervention phase of the study based on readiness of each ward. Each intervention ward entered into the intervention phase at different time points due to setting-specific factors such as time required to embed changes in CPOE systems (Appendix 1). The start of the intervention period was predetermined as the date of the first meeting of the QI team.
Enrollment
A Steering Committee comprising one lead from each site, research personnel, and quality improvement experts selected inpatient wards based on readiness for change (engaged mid-level inpatient ward managers and senior leadership) without similar concurrent initiatives. Site leads approached senior departmental and hospital leadership for approval and created local QI teams comprising frontline staff and key stakeholders. Timing of the start of the intervention phase was not randomized due to inherent variability in structures and processes specific to each hospital affecting timeliness of obtaining approvals for ethics review and CPOE order set changes, coordination of educational session, and rollout of sleep hygiene measures. The phases of the study and corresponding start dates were as follows: control period start dates ranged from May 2 to July 2, 2016; intervention phase start dates ranged from May 31, 2017, to September 6, 2017. All sites contributed intervention data for at least 16 months. Research ethics review boards at each participating hospital provided study approval. Timing and duration of study periods are displayed in Appendix 1.
Intervention
A prior single-center intervention formed the basis of a sedatives reduction bundle.11 The core components of the intervention emphasized system-level changes which are more durable than person-focused changes. The intervention bundle included:
-
(1)
Case-based interactive sessions (minimum of 2 cases per session) delivered to nurses and physicians on the harms of sedatives and emphasizing a non-pharmacological approach to in-hospital insomnia. These in-person sessions were conducted in small groups and repeated until all frontline staff were captured;
-
(2)
Pharmacist-enabled structured medication reviews to identify and remove new sedatives initiated for sleep and to reschedule non-essential medications scheduled for administration during sleep hours (period between 22:00 and 07:00). These reviews occurred at various frequencies depending on the ward but typically daily (Monday-Friday);
-
(3)
Identification and removal of “routine” nighttime sedative-hypnotic orders on admission order sets led by the local physician lead through standard hospital approval processes for order sets;
-
(4)
Implementation of sleep hygiene practices to create an environment conducive to sleep. This included engaging environmental services to reduce overnight noise and lighting, changes to nursing and pharmacy workflow to minimize nightly interruptions from clinical monitoring and non-essential medication administration (i.e., rescheduling medications to maintain a minimum of 6 h free of medication administration overnight), and education of nurses to first offer warm beverages, eye masks, and ear plugs to patients with difficulty sleeping;
-
(5)
Patient and caregiver engagement and education on sleep in hospital and alternatives to sedative-hypnotics using educational flyers posted and hand-outs provided throughout admission;
-
(6)
Incorporating sleep and sedative discussions into daily nursing huddle to empower nurses to trial non-pharmacologic strategies; and
-
(7)
Audit-feedback of sedative-hypnotic prescription rates among naïve patients to frontline teams which occurred 1–2 times per month.
Local QI teams were encouraged to modify and adapt the tools during intervention PDSA cycles; however, the 7 core components were considered critical to the intervention and were implemented in a standardized fashion across all participating sites. Examples of local adaptation included visible notes posted to ward telephones reminding clinicians to trial non-pharmacological sleep strategies prior to paging physicians for a sedative order. Some teams chose to display run charts of sedative-hypnotic prescription rates in a visible area on the ward while others discussed the results at weekly staff meetings. Posters, information sheets, and educational case study materials were shared. We measured sleep quality through patient surveys and the information was provided to care teams as motivation to maintain sleep hygiene practices on the ward. Small sample size precluded statistical analysis and interpretation.
Each hospital formed a local QI team comprised of a champion, medical lead with experience in quality improvement, nursing leadership, and a pharmacist. Teams met at least once monthly (more frequently at the start) and one member participated on the evaluation Steering Committee which also met once monthly.
Measures
The primary outcome measure was the proportion of sedative-hypnotic-naïve patients who received a new in-hospital sedative-hypnotic prescription for sleep. The proportion of patients prescribe new sedatives was collected as bi-weekly data. As part of national hospital accreditation standards, all inpatients underwent medication reconciliations using best possible medication history (BPMH) on admission which included documentation of any pre-hospital sedative-hypnotic use. The numerator was defined as sedative-hypnotic-naïve patients (no outpatient sedative-hypnotic use within 30 days of admission) discharged from a study inpatient ward AND prescribed a sedative for any indications of “sleep” or “insomnia” during the admission. If the indication for the sedative-hypnotic drug was not provided in CPOE, we assumed an as-needed order written for administration between 20:00 and 05:00 was for sleep. The denominator of sedative-hypnotic-naïve inpatients was determined by subtracting the number of patients prescribed pre-hospital sedative-hypnotics (home users) from the total number of patients discharged from the inpatient ward.
Secondary outcomes included in-hospital fall rates (number of in-hospital injurious falls per 1000 inpatient days), length of stay (LOS), mortality (defined as number of in-hospital deaths divided by total discharges per month), and proportion of patients prescribed other potentially harmful sedating drugs commonly used off-label for insomnia (i.e., quetiapine, olanzapine, and trazodone). Patient demographics, co-morbidities, and discharge locations were also extracted. Finally, to ensure that the primary outcome variable was not subject to changes in patient bed-days across sites, a secondary analysis was performed using total sedatives prescribed among naïve patients per 1000 inpatient days.
Data Collection
We included patients discharged from study inpatient wards during the study period. Patients with admission to a critical care ward were excluded as they did not receive the intervention. A single patient could have had more than one hospital admission during the study period. Sedative-hypnotic prescriptions were electronically extracted from hospital pharmacy databases along with indications provided at the time of ordering and whether prescriptions were one-time, as-needed (PRN), and/or scheduled. Sedative-hypnotic-naïve status was determined through manual chart review of BPMH.
One abstractor at each site collected data into a standardized Microsoft Excel spreadsheet. A second abstractor verified a minimum of 30% of data entries with an agreement of 97% between study personnel with a kappa of 0.94. When discrepancies arose, a research coordinator adjudicated and finalized data elements.
Statistical Analysis
For each outcome, generalized linear mixed effects regression (GLMER) was used to fit a piecewise regression model using R package lme4. The GLMER framework was selected because it affords estimation of overall level change and overall temporal trend within each intervention phase, while accounting for unit-level heterogeneity in both absolute level of the outcome and trajectory over time.13 The binary outcomes (percent new sedatives, percent mortality, and percent prescribed other sedative medications) were modeled using a binomial likelihood and logit link function; and fall rate, using a Poisson likelihood and log link function. The fixed effects are a level change between intervention phases, and temporal trend within each phase. A random slope and random intercept are included for each unit. An additional random intercept is provided for month name (January, February, et cetera) to address seasonality and to reduce potential bias introduced by the staggered intervention start dates and variation across units in the length of follow-up. Each model is fit twice, as the desired estimates required alternate parameterizations of time. We report estimates, 95% confidence intervals, and Wald’s test p-values for the following quantities:
-
1.
Level change between the end of the control phase and the start of the intervention phase, to assess evidence of an interruption at the start of the intervention;
-
2.
Level change between the end of the control phase and month 16 of the intervention phase (all units contributed data for at least 16 months) to assess evidence of long-term change in outcome;
-
3.
Control phase slope, to assess evidence that outcomes were changing over time before the intervention;
-
4.
Intervention phase slope, to assess evidence that the outcomes were changing over time in the intervention phase
-
5.
The change in slope between the control phase and the intervention phase, to assess evidence of a change in trend between the phases.
For comparability, all estimates involving trend are for a 1-month increase in time. Unadjusted variables (diagnoses and co-morbidities) were described using chi-squared tests and the remainder using t-tests. The analysis was performed using R version 3.2.6. (Free Software Foundation, Boston, MA, USA).
RESULTS
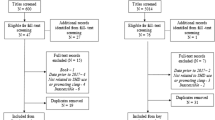
During the study period, 8,970 and 10,120 inpatient cases were discharged from the control and intervention cohorts, respectively. Corresponding sedative-naïve discharges were 8,046 during the control and 9,003 during the intervention periods. Patient characteristics and unadjusted outcomes are displayed in Tables 1 and 2, respectively. Mean age was 65.71 (SD 7.24), 49.43% were female (SD 9.45), and top admission diagnoses were cardiovascular, heart failure, gastrointestinal disease, pneumonia, diabetes, and chronic obstructive lung disease. There were no differences between the control and intervention groups in co-morbidities of delirium, dementia, stroke, and psychiatric diagnoses. Unadjusted proportion of new sedative-hypnotic initiation decreased from 15.48% in the control period to 9.08% in the intervention period (p<0.001) (Table 2). Adjusted odds ratio for start of intervention versus end of control period was 0.814 (95% CI: 0.667–0.993, p=0.042) (Table 3). Odds of sedative-hypnotic use did not change significantly in the control period trend, whereas in the intervention period, the odds ratio of sedative-hypnotic use per month was 0.972 (95% CI: 0.957–0.986, p<0.001). Ward-specific outcomes are shown in Appendices 3 and 4.
There were no differences in the secondary outcomes of mortality (adjusted OR 1.089; 95% CI: 0.786–1.508, p=0.608), falls (adjusted rate ratio 0.819; 95% CI: 0.625–1.073, p=0.148), or other sedating drug prescriptions (adjusted OR 1.046; 95% CI: 0.873–1.252, p=0.627) (Table 3).
We conducted a secondary analysis of the total number of sedatives prescribed to naïve patients per 1000 inpatient days. Unadjusted prescribing rate was 65.34 (SD 27.49) in the control versus 62.58 (SD 34.33) in the intervention group (p=0.526). The GLMER adjusted rate ratio is significant comparing the start of intervention versus the end of the control periods (RR 0.73, 95% CI: 0.582–0.917, p=0.007) and month 16 intervention versus end of control (RR 0.803, 95% CI: 0.739-0.872, p<0.001)
DISCUSSION
A sedative-hypnotic reduction intervention bundle was associated with a statistically significant reduction in-hospital sedative-hypnotic prescribing among naïve patients. The reduction was sustained at all 5 hospitals for more than 16 months. Incorporating this intervention in hospital settings can reduce low-value care and have significant positive impact on patient safety.
Numerous studies examined reducing sedative-hypnotic use among inpatients as either a primary or secondary endpoint in the context of improving sleep in hospital or overall appropriate medication use.10 The majority of studies described single-centered observational before-after designs and single-faceted intervention types.14,15,16,17,18 One Australian study set in long-term care facilities implemented a similar multi-faceted intervention and found a comparable effect size with a benzodiazepine prescription prevalence decreasing from 31.8 to 26.9% (p<0.005) over a 26-week period.19 A subsequent 12-month follow-up study found a sustained 25% reduction in mean daily diazepam equivalent dose (p<0.02).20 Another multi-centered Australian study implemented audit and feedback method among 9 hospitals and found no significant reduction in initiation of benzodiazepine14,15,16,17,18,21 A multi-centered Swiss study examined the impact of education and audit-feedback on benzodiazepine prescriptions on discharge among naïve patients and found a reduction from 7.2 to 5.5%.22 However, the intervention was limited to education and audit-feedback without systems-focused components such as order set or CPOE changes. The strengths of our study lie in a comprehensive intervention utilizing effective and durable systems-focused components (e.g., order set changes, pharmacy structured medication reviews), the long duration of the intervention period (16 months), multi-centered design, and use of patient surveys to refine the intervention.
Several limitations of this study merit discussion. First, wards were unable to simultaneously enter into the intervention phase due to pragmatic reasons. The benefits of allowing wards to choose the time to implement the intervention included ensuring completion of necessary components such as securing senior leadership support, incorporating CPOE enabled changes and ability to conduct educational outreach sessions of all staff. Second, results may not be generalizable as participating hospitals were all large urban teaching hospitals affiliated with a university with an established record of resource stewardship.23 However, by including diverse inpatient populations (such as medical and surgical patients), our cohort and environment are reasonably representative of a typical inpatient ward. Third, the control rate of 15.48% of new sedative-hypnotic starts may appear to be lower than that previously reported and may be interpreted as a pre-existing culture of change readiness and/or stewardship.11 Fourth, there was no improvement in measured clinical outcomes (i.e., falls, LOS, mortality) and we measured prescriptions rather than actual drug administration. However, multiple other factors beyond the scope of this study impact these clinical outcomes and reducing sedative-hypnotic prescriptions alone is unlikely to impact measures such as falls, LOS, and mortality. Fifth, we were unable to assess for reductions in dosing range or the numbers of doses or post-discharge prescriptions. However, we achieved our goal to reduce low-value care and a patient’s exposure to harm in pursuit of the Quadruple Aim of healthcare. Sixth, we did not include all possible sedating drugs that might be used off-label for sleep although we were reassured that there was no increase patients being prescribed in the drugs we measured. Seventh, large differences in the sample size of the sleep survey data preclude statistical analyses of sleep experience. Last, no adjustment was made for patient-level covariates although descriptive statistics did not reveal significant differences in co-morbidities that might influence sedative prescribing (i.e., dementia, delirium, or psychiatric diagnoses) between the control and intervention groups.
CONCLUSION
A sedative-hypnotic reduction bundle resulted in fewer patients prescribed sedative-hypnotics and reduced exposure to possible adverse events. The intervention components can be implemented in any inpatient setting. Institutions should consider adopting this intervention to reduce patient exposure to unnecessary harms of sedative-hypnotics and to improve the sleep environment in hospital.
References
Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: Meta-analysis of risks and benefits. BMJ. 2005;331(7526):1169. https://doi.org/10.1136/bmj.38623.768588.47.
Bell CM, Fischer HD, Gill SS, et al. Initiation of benzodiazepines in the elderly after hospitalization. J Gen Intern Med 2007;22(7):1024-1029. https://doi.org/10.1007/s11606-007-0194-4.
Gillis CM, Poyant JO, Degrado JR, Ye L, Anger KE, Owens RL. Inpatient pharmacological sleep aid utilization is common at a tertiary medical center. J Hosp Med (Online) 2014;9(10):652-657. https://doi.org/10.1002/jhm.2246.
Pek EA, Remfry A, Pendrith C, Fan-Lun C, Bhatia S, Soong C. High prevalence of inappropriate benzodiazepine and sedative hypnotic prescriptions among hospitalized older adults. J Hosp Med. 2017;12(5):310-316. https://doi.org/10.12788/jhm.2739.
Kang D-Y, Park S, Rhee C-W, et al. Zolpidem use and risk of fracture in elderly insomnia patients. J Prev Med Public Health 2012;45(4):219-226. https://doi.org/10.3961/jpmph.2012.45.4.219.
Lyons PG, Snyder A, Sokol S, Edelson DP, Mokhlesi B, Churpek MM. Association between opioid and benzodiazepine use and clinical deterioration in ward patients. J Hosp Med (Online). 2017;12(6):428-434. https://doi.org/10.12788/jhm.2749.
Cumbler E, Guerrasio J, Kim J, Glasheen J. Use of medications for insomnia in the hospitalized geriatric population. J Am Geriatr Soc 2008;56(3):579-581. https://doi.org/10.1111/j.1532-5415.2008.01598.x.
Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: Five opportunities for improved healthcare value. J Hosp Med (Online) 2013;8(9):486-492. https://doi.org/10.1002/jhm.2063.
Choosing Wisely Canada. Five things physiciains and patients should question: Canadian Geriatrics Society. http://www.choosingwiselycanada.org/recommendations/geriatrics/. Accessed 8 Dec 2021.
Soong C, Burry L, Cho HJ, et al. An Implementation Guide to Promote Sleep and Reduce Sedative-Hypnotic Initiation for Noncritically Ill Inpatients. JAMA Intern Med 2019;179(7):965-972. https://doi.org/10.1001/jamainternmed.2019.1196.
Fan-Lun C, Chung C, Lee EHG, et al. Reducing unnecessary sedative-hypnotic use among hospitalised older adults. BMJ Qual Saf 2019;28(12):1039-1045. https://doi.org/10.1136/bmjqs-2018-009241.
IHI. How to improve. IHI. http://www.ihi.org/resources/Pages/HowtoImprove/default.aspx. Accessed 8 Dec 2021.
Fitzmaurice, G. M. & Ravichandran, C. A primer in longitudinal data analysis. Circulation 118, 2005–2010 (2008).
Chung S, Youn S, Park B, Lee S, Kim C. A sleep education and hypnotics reduction program for hospitalized patients at a general hospital. Psychiatry Investig 2018;15(1):78-83. https://doi.org/10.4306/pi.2018.15.1.78.
Bartick MC, Thai X, Schmidt T, Altaye A, Solet JM. Decrease in as-needed sedative use by limiting nighttime sleep disruptions from hospital staff. J Hosp Med (Online) 2010;5(3):E20-E24. https://doi.org/10.1002/jhm.549.
McDowell JA, Mion LC, Lydon TJ, Inouye SK. A nonpharmacologic sleep protocol for hospitalized older patients. J Am Geriatr Soc 1998;46(6):700-705.
Browne C, Kingston C, Keane C. Falls prevention focused medication review by a pharmacist in an acute hospital: Implications for future practice. Int J Clin Pharm 2014;36(5):969-975. https://doi.org/10.1007/s11096-014-9980-3.
Agostini JV, Zhang Y, Inouye SK. Use of a computer-based reminder to improve sedative-hypnotic prescribing in older hospitalized patients. J Am Geriatr Soc 2007;55(1):43-48. https://doi.org/10.1111/j.1532-5415.2006.01006.x.
Westbury J, Jackson S, Gee P, Peterson G. An effective approach to decrease antipsychotic and benzodiazepine use in nursing homes: The RedUSe project. Int Psychogeriatr 2010;22(1):26-36. https://doi.org/10.1017/S1041610209991128.
Westbury J, Tichelaar L, Peterson G, Gee P, Jackson S. A 12-month follow-up study of “RedUSe”: A trial aimed at reducing antipsychotic and benzodiazepine use in nursing homes. Int Psychogeriatr 2011;23(8):1260-1269. https://doi.org/10.1017/S1041610211000421.
Elliott RA, Woodward MC, Oborne CA. Improving benzodiazepine prescribing for elderly hospital inpatients using audit and multidisciplinary feedback. Intern Med J 2001;31(9):529-535.
Del Giorno R, Greco A, Zasa A, et al. Combining prescription monitoring, benchmarking, and educational interventions to reduce benzodiazepine prescriptions among internal medicine inpatients; a multicenter before and after study in a network of Swiss Public Hospitals. Postgrad Med 2018;130(7):627-636. https://doi.org/10.1080/00325481.2018.1504594.
Levinson W, Kallewaard M, Bhatia RS, et al. “Choosing Wisely”: A growing international campaign. BMJ Qual Saf 2015;24(2):167-174. https://doi.org/10.1136/bmjqs-2014-003821.
Acknowledgements
We would like to thank the following for their contributions to this study: the nursing and interprofessional healthcare teams at Sinai Health, Unity Health, Sunnybrook Health Sciences Center and University Health Network for their support and efforts in reducing sedative-hypnotics among inpatients.
Funding
This study was supported by the Innovation Fund of the Alternative Funding Plan for the Academic Health Sciences Centres of Ontario.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no relevant conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soong, C., Ethier, C., Lee, Y. et al. Reducing Sedative-Hypnotics Among Hospitalized Patients: a Multi-centered Study. J GEN INTERN MED 37, 2345–2350 (2022). https://doi.org/10.1007/s11606-021-07292-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-07292-5