Abstract
A 46-year-old woman was type 1 diabetes diagnosed at the age of 9 who had previously been on an insulin pump. Other co-morbidities included CKD IV, HTN, and hypothyroidism. She presented with hyperglycemia of 400 mg/dl and fluid retention. Her GFR had decreased to 13. Her physical exam was notable for respiratory distress and anasarca. She failed to respond to aggressive IV diuresis and urgent hemodialysis was initiated. The patient had been lost to outpatient follow-up for a year. She had been co-managed by an endocrinologist and a primary care physician but had stopped going to her endocrinologist over a year ago due to inability to afford the co-pays. She subsequently lost her insurance and had to pay out of pocket for her insulin; at this point, she decided to stop seeing her PCP and began to ration her insulin. Due to social stigma, she did not mention her financial issues to her healthcare providers. After identifying these challenges, we decided to start her on a more affordable regimen of NPH insulin. Through social work assistance, we were able to obtain a charity hemodialysis chair and discharge her home. She applied to Medicaid. Healthcare expenditure with regard to diabetes rose to $327 billion from $245 billion in 2012. The price of insulin has continued to increase even after the drug’s patent has expired due to the combination of FDA requirements, a monopoly in the insulin market, and the lack of federal price controls and Pharmacy Benefits Managers. The high out of pocket costs for insulin has led to many instances of insulin rationing among both uninsured and insured. This led to death in some cases as well as poorly controlled diabetes with increased complications and mortality as in our case. We present a case report and narrative review on insulin affordability.
Similar content being viewed by others
CASE PRESENTATION
ET is a 46-year-old woman with a poorly controlled type one diabetes (T1D) diagnosed at 9 years, diabetic nephropathy, diabetic peripheral neuropathy, essential hypertension, and hypothyroidism. She presented in the emergency department with respiratory distress, anasarca, and a weight gain of 35 lbs over the last couple of weeks. Her physical exam was pertinent for an elevated blood pressure of 193/97 mmHg, with ascites and bilateral lower extremity pitting edema. Laboratory workup revealed hyperglycemia with serum glucose of 400 mg/dl, GFR of 13 mL/min/1.73 m2, and BUN of 49. Her last HbA1c a year prior to presentation was 8.8%. Her chest x-ray showed bilateral pleural effusions. She failed to respond to aggressive intravenous diuresis and urgent hemodialysis had to be initiated. In subsequent interviews during this hospitalization, ET disclosed that her diabetes had previously been managed with an insulin pump under the joint care of her endocrinologist and primary care provider (PCP). However, she had not seen her endocrinologist in over a year due to losing her job and being unable to afford the co-payments. ET subsequently lost her insurance and began to pay out-of-pocket for insulin. Eventually, she decided to stop seeing her PCP and began to ration her insulin. She did not bring up her financial difficulties to healthcare providers for fear of stigma. After identifying these challenges, we decided to start the patient on a more affordable regimen of Neutral Protamine Hagedorn (NPH) insulin combined with regular insulin. After starting hemodialysis, her blood pressure improved and she was able to stop all antihypertensive medications. Through social work assistance, we were safely discharge her to home with outpatient hemodialysis provided through the charity of a local dialysis center. In the months following this hospitalization, ET would apply for Medicare and social security disability and be hospitalized for diabetic ketoacidosis.
INTRODUCTION
A Lifesaving Drug
Before the discovery of insulin by Banting, Collip, and Best in 1922, the diagnosis of TID was a death sentence with patients given weeks to months to live.1 In order to make insulin available to diabetics who required insulin, the patent was sold to the University of Toronto for only $1.2 Nearly 100 years later, at least 12 individuals have experienced untimely deaths since 2017 from diabetes either due to insulin rationing or the complete lack of insulin.3,4,5,6 Numerous other untold individuals, such as our patient above, have likely developed co-morbidities in which insulin and optimal diabetic control can delay.7 Furthermore, the plethora of social media sites such as GoFundMe with requests to help pay for insulin or other diabetic supplies has cried attention to the problem of insulin affordability for insulin-dependent diabetics in the USA. Despite this attention, patients with diabetes continue to face the dire prospect of choosing between insulin and paying for other necessities.8
Diabetes is a chronic disease with significant but often preventable morbidity and mortality. According to the American Diabetes Association (ADA), more than 30 million Americans have some form of diabetes. Of this number, approximately 7.4 million use at least one formulation of insulin.8 Insulin is a necessary treatment in many hyperglycemic conditions, including T1D, a significant percentage of type 2 diabetics (T2D), and gestational diabetes. Per the 2019 ADA recommendations, a combination of multiple daily insulin injections or continuous subcutaneous administration through an insulin pump provides optimal safety and efficacy for T1D patients.9 For patients with T2D, insulin may be required to achieve target glycated hemoglobin (HbA1c) levels in addition to other diabetic agents including metformin, sulfonylureas, thiozolidinediones, sodium glucose transporter 2 inhibitors, GLP-1 receptor agonists, and DPP-4 inhibitors.10
Scope of the Problem
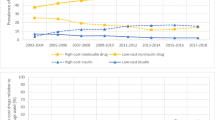
The number of patients diagnosed with diabetes in the USA has risen substantially since the 1990s. In this same period, the price of insulin has outstripped inflation. A 2016 study found that insulin prices in the USA tripled from 2002 to 2013. In this same period, prices for non-insulin anti-diabetic drugs fell.11 In 2017, people with diabetes experienced annual healthcare expenditure approximately 2.3 times greater than people without diabetes.12 The added financial burden primarily impacts those on Medicare or without insurance, though patients with private insurance are not spared from high prices.13 As such, rising insulin prices in the setting of a growing diabetic population can prevent many patients from having their diabetes appropriately treated.
Roberts describes how “back in 1996, when Eli Lilly’s Humalog first came out, the price for a 1-month supply of insulin was $21. As of 2001, that exact vial’s price increased by $14 to $35. Now, in 2019, that vial is said to be around $275.”14 Please see Figure 1 for common current insulin prices.15 The list price of insulin and spending on insulin by pharmacies, insurance, Medicare, and out-of-pocket expenses have increased 10–17% from 2012 to 2016.16 This increase is significantly higher than the rate of inflation at 2.2%, as well as the increase in rates of medical expenditures (3.8%) and other drugs (2.8%). Manufacturers are gradually introducing innovations to their insulin preparations.17 Manufacturers have also increased the prices of their older formulations, preventing these formulations from becoming affordable for patients over time.18
The Role of Pharmaceutical Benefits Managers
The exorbitant cost of insulin is more complicated than tight control of the market by the three large insulin manufacturers Lilly, Novo Nordisk, and Sanofi, whose increase in net price is considerably lower than the increase in list price due to contractual agreements and rebates. Pharmaceutical Benefits Managers (PBMs) work with pharmaceutical manufacturers and wholesalers to lower cost for employee benefits companies and insurance companies. Three PBMs, CVS Caremark, Express Scripts, and Optum Rx control about 76% of the market.19 The participation of PBMs may benefit well-insured individuals but leaves under-insured and uninsured patients paying the extremely high list price for their medication’s PBMs do not directly work for the patient, nor can their role be easily understood by the patient or their physician. This system encourages higher list prices since PBMs can then demonstrate higher rebates to insurance companies and higher handling costs to wholesalers. Insurers (e.g., Cigna) have introduced plans which limit out of cost payments for their patients; however, employers can opt out of these plans.
Complex Regulation
Scientifically speaking, insulin has always been a biologic because it was initially manufactured from pigs and cows, and in 1982 became the first recombinant human product. However, alongside a small subset of biologics, insulin was designated as a drug and regulated under the statute for drugs, the Federal Food, Drug, and Cosmetic Act (FD&C Act), rather than a biologic under the Public Health Service Act (PHSA). Since drugs and biologics are regulated under different statutes, they possess varying protections and are subject to distinct rules. The classification of insulin as a drug prevented it from being authorized under the PHSA and created barriers to seeking approval and developing biosimilars. Biologics are produced by living cell cultures, have complex heterogeneous structures, and can be unstable and sensitive to external conditions. Biosimilars are compounds that have to demonstrate no clinically meaningful differences in safety, purity, and potency to the reference biologic product. They must demonstrate the same amino acid sequences and action mechanism but may vary in other minor aspects. They can be considered the “generics” of biologics. In 2009, the Biologics Price Competition and Innovation Act created a process by which biosimilars could hit the market. The process took over a decade, but on March 23, 2020, insulin transitioned from initially approved under the FD&C Act to being regulated by the PHSA and opened a pathway for biosimilar insulins to be introduced to the market.20 Please see Table 1. This regulation may eventually encourage competition by relaxing data acquisition requirements for generic formulations that share the same chemical properties as vetted brand-name formulations.18 However, all current insulins were created under a Standalone Biological License Application rather than as designated biologics or biosimilars and cannot be considered interchangeable. Additionally, insulin delivery devices such as pumps have only been studied with brand name insulins. For T1D patients who become accustomed to these devices, it can be very difficult to switch to injections of NPH and regular insulin. The optimal treatment of diabetics often requires a combination of insulin as well as delivery and monitoring devices which have shown favorable effects on glycemic control.21 It is currently unclear when biosimilars can be licensed with these devices.
A Health Disparity
The cost of insulin is likely to have a higher impart on minorities. Diabetes control evaluation with HbA1C can vary depending on racial/ethnic groups. A study by Smalls et al. examined the differences in HbA1C among racial groups and found that racial minorities have higher values than non-Hispanic Whites. Shivani et al.22 addressed ethnic disparities in diabetes technologies and found non-Hispanic Whites to have significantly higher insulin pump and continuous glucose monitoring (CGM) use (72%) compared with non-Hispanic Blacks (18%). The reason for such disparity was explained as a combination of socioeconomical status, health literacy, and health care provider racism and implicit bias.23
New Legislations and Plans
Currently, individual states negotiate purchasing contracts based on drug pricing benchmarks that may not accurately reflect what pharmacies pay to acquire drugs.24 Ten states have now passed legislation that will reduce the out-of-pocket costs or co-pay costs for insulin, and many other states have legislation pending.25 However, none of these will start before 2021, and most will not benefit uninsured patients or those with high deductible plans that require paying the entire costs of insulin before qualifying for a price reduction. Additionally, many diabetics utilize two different insulin formulations daily (long-acting insulin and short-acting insulin) requiring paying two different co-payments. Medicare has proposed a pilot program in 2021 to limit insulin out of pocket costs to 35 dollars per month but will only cover patients on enhanced Medicare drug plans, which require higher monthly costs.26 Manufacturers have also introduced plans to reduce out of pocket costs for patients such as Novocares27 and are introducing lower price alternatives for their current formulations. Former President Trump’s executive action on July 24, 2020, will help some patients who obtain insulin through Federally Qualified Health Center which are now required to pass 340B savings on to their patients28 but this executive order did not provide a timeframe or an implementation plan. Consequently, insulin access is a complex patchwork that is hard to navigate and is likely to leave many vulnerable diabetics with limited access to sufficient insulin.
In response to the attention this issue received, several legislative attempts to address it have been proposed in the United States Congress. Most recently, among these is the Insulin Price Reduction Act29 and the Emergency Access to Insulin Act in 2019. This last Act seeks to increase insulin access by having state-level assistance programs temporarily provide insulin for patients in need. This Act also seeks to check the influence of insulin manufacturers by reducing exclusivity periods, thereby allowing generic formulations to enter the market more rapidly.17 However, neither of these acts have moved forward in Congress.
At the provider level, medication affordability should be addressed at each visit. However, there are no specific guidelines on how to safely navigate this area without creating stigma. Utilizing the questionnaire developed by AAFP (EveryONE project™) can elucidate patients at risk and help point to resources.30 In order to decrease stigma, questions about medication affordability may be better asked as part of a screening tool, similar to questions about smoking, falls, and depression. Utilizing these tools can engage the physician and the patient in a discussion about resources, options, and the need for advocacy.
CONCLUSION
One hundred years after the discovery of insulin and despite the health equity plan of the Banting, Best, and Collip, many vulnerable diabetics such as ET continue to struggle to afford insulin. Complex governmental regulations, the lack of generic designations, the power of insulin manufacturers, and Pharmaceutical Benefits Managers (PBMs) as well as access to comprehensive health insurance have all contributed to this excess vulnerability. Patients with lower and unstable incomes are disproportionately affected by socioeconomical status, health literacy, and racial and ethnic discrimination as burdens of diabetes, often leading to cases like ET’s. Before losing her connection to her endocrinologist and PCP, ET struggled to keep up with the price increases of insulin for 5 years. These circumstances created a situation that forced ET to ration her insulin for a year. Her kidney failure was ultimately secondary to limited medication access and fear of stigma. Ironically, federal and state assistance is now paying for dialysis when a much cheaper option—paying for insulin—could have prevented or delayed dialysis.
Healthcare providers have an obligation to be aware of the socioeconomic contexts of their patients and attempt to individualize treatment plans by taking these contexts into account. In particular, medication affordability needs to be addressed with sensitivity at every attended or missed medical encounter to prevent progression in a patient’s condition. Making time to inquire about the patient’s ability to afford each medication, utilizing screening tools during each visit, and offering training in patient-centered models can address this disparity. Relief for vulnerable patients may take many forms such as hospital assistance programs, legislative reforms, and manufacturer’s assistance programs. All of these may be intermittently needed to achieve the best clinical outcomes possible for people like ET until more comprehensive reform is achieved. Unfortunately, this patchwork of assistance is constantly changing and continues to leave patients vulnerable to preventable co-morbidities and premature death.
References
University of Toronto. The Discovery and Early Development of Insulin. The Discovery and Early Development of Insulin. https://insulin.library.utoronto.ca/. Accessed 5 July 2020.
bantinghouse. Insulin Patent Sold for $1. Banting House. Published December 14, 2018. https://bantinghousenhsc.wordpress.com/2018/12/14/insulin-patent-sold-for-1/. Accessed 13 May 2020.
Smith-Holt N. My son died from rationing insulin. Democrats’ drug pricing plan still wouldn’t help him. USA TODAY. https://www.usatoday.com/story/opinion/voices/2019/12/10/insulin-rationing-drug-prices-death-health-insurance-column/2629757001/. Accessed 25 May 2020.
Insulin’s High Cost Leads To Lethal Rationing. NPR.org. https://www.npr.org/sections/health-shots/2018/09/01/641615877/insulins-high-cost-leads-to-lethal-rationing. Accessed 25 May 2020.
Brooks: As talks at State Capitol derail, Minnesota diabetics pay price - StarTribune.com. https://www.startribune.com/as-talks-derail-minnesota-diabetics-pay-price/565382102/. Accessed 5 July 2020.
Jones S. How Trumpcare’s Failure Sets the Stage for Single-Payer. New Repub. Published online March 28, 2017. https://newrepublic.com/article/141651/trumpcares-failure-sets-stage-single-payer. Accessed 5 July 2020.
Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metab Disord. 2013;12:14. https://doi.org/10.1186/2251-6581-12-14.
Cefalu WT, Dawes DE, Gavlak G, et al. Insulin Access and Affordability Working Group: Conclusions and Recommendations. Diabetes Care. 2018;41(6):1299-1311. https://doi.org/10.2337/dci18-0019.
Association AD. 8. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S73-S85. https://doi.org/10.2337/dc18-S008.
Beran D, Hirsch IB, Yudkin JS. Why Are We Failing to Address the Issue of Access to Insulin? A National and Global Perspective. Diabetes Care. 2018;41(6):1125-1131. https://doi.org/10.2337/dc17-2123.
Hua X, Carvalho N, Tew M, Huang ES, Herman WH, Clarke P. Expenditures and Prices of Antihyperglycemic Medications in the United States: 2002–2013. JAMA. 2016;315(13):1400-1402. https://doi.org/10.1001/jama.2016.0126.
Dall TM, Yang W, Gillespie K, et al. The Economic Burden of Elevated Blood Glucose Levels in 2017: Diagnosed and Undiagnosed Diabetes, Gestational Diabetes Mellitus, and Prediabetes. Diabetes Care. 2019;42(9):1661-1668. https://doi.org/10.2337/dc18-1226.
Hirsch IB. Insulin in America: A Right or a Privilege? Diabetes Spectr Publ Am Diabetes Assoc. 2016;29(3):130-132. https://doi.org/10.2337/diaspect.29.3.130.
The Deadly Costs of Insulin. AJMC. https://www.ajmc.com/contributor/danielle-roberts/2019/06/the-deadly-costs-of-insulin. Accessed 26 May 2020.
Lee B. How much Does Insulin Cost? Here's How 27 brands and generics compare – GOODRX. 2020. Retrieved February 23, 2021, from https://www.goodrx.com/blog/how-much-does-insulin-cost-compare-brands/.
The U.S. Insulin Crisis — Rationing a Lifesaving Medication Discovered in the 1920s | NEJM. Accessed 5 July 2020. https://www.nejm.org/doi/full/10.1056/NEJMp1909402.
Why Is There No Generic Insulin? Historical Origins of a Modern Problem | NEJM. https://www.nejm.org/doi/full/10.1056/NEJMms1411398. Accessed 5 July 2020.
Luo J, Avorn J, Kesselheim AS. Trends in Medicaid Reimbursements for Insulin From 1991 Through 2014. JAMA Intern Med. 2015;175(10):1681-1686. https://doi.org/10.1001/jamainternmed.2015.4338.
Fein AJ. Drug Channels: CVS, Express Scripts, and the Evolution of the PBM Business Model. Drug Channels. Published May 29, 2019. https://www.drugchannels.net/2019/05/cvs-express-scripts-and-evolution-of.html. Accessed 5 July 2020.
George K, Woollett G. Insulins as Drugs or Biologics in the USA: What Difference Does it Make and Why Does it Matter? BioDrugs. 2019;33(5):447-451. https://doi.org/10.1007/s40259-019-00374-1.
Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012;157(5):336-347. https://doi.org/10.7326/0003-4819-157-5-201209040-00508.
Smalls BL, Ritchwood TD, Bishu KG, Egede LE. Racial/Ethnic differences in glycemic control in older adults with type 2 Diabetes: United States 2003–2014. Int J Environ Res Publ Health. 2020;17(3):950. https://doi.org/10.3390/ijerph17030950.
Agarwal S, Schechter C, Gonzalez J, Long JA. Racial–ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diab Technol Ther. 2020;23(4). https://doi.org/10.1089/dia.2020.0338.
Bruen B. May 23 KYP. Paying for Prescribed Drugs in Medicaid: Current Policy and Upcoming Changes. KFF. 2014. Published May 23, 2014. Accessed July 5, 2020. https://www.kff.org/medicaid/issue-brief/paying-for-prescribed-drugs-in-medicaid-current-policy-and-upcoming-changes/.
Eight States Pass Legislation to Place Caps on Insulin Price; Five More Await Ruling. diaTribe. Published April 20, 2020. https://diatribe.org/eight-states-pass-legislation-place-caps-insulin-price-five-more-await-ruling. Accessed 6 July 2020.
Thomas K. Insulin Costs May Be Capped in a Medicare Program. The New York Times. https://www.nytimes.com/2020/03/11/health/insulin-drug-prices.html. Published March 11, 2020. Accessed 7 July 2020.
Novo Nordisk’s new insulin affordability offerings now available in the US. http://www.novonordisk-us.com/media/news-releases.html. Accessed 5 July 2020.
U.S. Department of Health and Human Services. Trump Administration Announces Historic Action to Lower Drug Prices for Americans. 2020. Retrieved September 27, 2020, from https://www.hhs.gov/about/news/2020/07/24/trump-administration-announces-historic-action-lower-drug-prices-americans.html.
Bipartisan Senate Bill Seeks to Roll Back a Decade’s Worth of Insulin Price Hikes. https://www.ajmc.com/focus-of-the-week/bipartisan-senate-bill-seeks-to-roll-back-a-decades-worth-of-insulin-price-hikes. Accessed 26 May 2020.
O'Gurek D, Henke C. A practical approach to screening for social determinants of health. 2018. Retrieved February 23, 2021, from https://www.aafp.org/fpm/2018/0500/p7.html.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors of this manuscript have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arce Gastelum, A., Maraqa, S., Marquez Lavenant, W.A. et al. Who Will Be Responsible for the Dialysis Bill? A Case Report and Narrative Review of Insulin Affordability 100 Years After the Discovery of Insulin. J GEN INTERN MED (2021). https://doi.org/10.1007/s11606-021-06886-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11606-021-06886-3