Abstract
Background
Implementation methods of risk-stratified cancer screening guidance throughout a health care system remains understudied.
Objective
Conduct a preliminary analysis of the implementation of a risk-stratified prostate cancer screening algorithm in a single health care system.
Design
Comparison of men seen pre-implementation (2/1/2016–2/1/2017) vs. post-implementation (2/2/2017–2/21/2018).
Participants
Men, aged 40–75 years, without a history of prostate cancer, who were seen by a primary care provider.
Interventions
The algorithm was integrated into two components in the electronic health record (EHR): in Health Maintenance as a personalized screening reminder and in tailored messages to providers that accompanied prostate-specific antigen (PSA) results.
Main Measures
Primary outcomes: percent of men who met screening algorithm criteria; percent of men with a PSA result. Logistic repeated measures mixed models were used to test for differences in the proportion of individuals that met screening criteria in the pre- and post-implementation periods with age, race, family history, and PSA level included as covariates.
Key Results
During the pre- and post-implementation periods, 49,053 and 49,980 men, respectively, were seen across 26 clinics (20.6% African American). The proportion of men who met screening algorithm criteria increased from 49.3% (pre-implementation) to 68.0% (post-implementation) (p < 0.001); this increase was observed across all races, age groups, and primary care clinics. Importantly, the percent of men who had a PSA did not change: 55.3% pre-implementation, 55.0% post-implementation. The adjusted odds of meeting algorithm-based screening was 6.5-times higher in the post-implementation period than in the pre-implementation period (95% confidence interval, 5.97 to 7.05).
Conclusions
In this preliminary analysis, following implementation of an EHR-based algorithm, we observed a rapid change in practice with an increase in screening in higher-risk groups balanced with a decrease in screening in low-risk groups. Future efforts will evaluate costs and downstream outcomes of this strategy.
Similar content being viewed by others
INTRODUCTION
Among men, prostate cancer is the most common non-skin cancer and the second most common cause of cancer-specific mortality.1, 2 Though prostate cancer is one of only five cancers in which randomized controlled trials (RCTs) have shown that screening leads to a reduction in cancer-specific mortality,3, 4 controversy has enshrouded prostate-specific antigen (PSA)–based screening for the past two decades.5, 6 In particular, there has been concern that harms of screening, including the resultant increase in diagnosis and overtreatment, outweigh the potential benefits.7 Indeed, in 2012, the US Preventive Services Task Force (USPSTF) recommended against PSA-based screening8 while other guideline groups, including the American Cancer Society (ACS)9 and the National Comprehensive Cancer Network (NCCN),10 recommended a risk-stratified approach centered on informed decision-making.
In the ensuing years, PSA-based screening rates among men 50 years and older decreased from 41% in 2008 to 31% in 2013 with a resultant decrease in the incidence of local- and regional-stage disease.11 However, the incidence of distant stage disease increased 1.4% per year from 2008 to 2013 for men ages 50 to 74.12 With new evidence based upon longer follow-up of the RCTs,13, 14 the USPSTF changed to a recommendation of informed decision-making regarding screening among men 55 to 69 years.15 While the task force noted the increased incidence and risk of advanced prostate cancer among African American men and those with a family history, no special considerations were given additional weight. Importantly, African American men have not been sufficiently studied in RCTs,3, 16 despite being disproportionately impacted by lethal prostate cancer.1, 17 In contrast to the USPSTF, the ACS recommends a risk-stratified approach with early screening in high-risk individuals (African Americans, men with a first-degree relative diagnosed with prostate cancer prior to age 65) and in men age 50 and older who have a life expectancy of at least 10 years.18 In addition to using a risk-stratified approach with family history and race, the NCCN guideline19 incorporates the findings that a midlife baseline PSA for a man in his forties predicts future risk of prostate cancer death or metastases.20,21,22 Subsequent monitoring is based upon the age and the PSA level, along with the digital rectal examination.
To provide a standardized approach for clinicians at Duke Health, where 21% of men are African Americans, a multi-disciplinary group from Duke Health, Duke Primary Care (DPC) and the Duke Cancer Institute (DCI), developed a risk-stratified prostate cancer screening algorithm incorporating an informed decision process (Supplementary Material).23 This algorithm was coupled with the development of a risk-stratified treatment pathway based on two paradigms. First, there is insufficient evidence that radical prostatectomy reduces prostate-specific cancer mortality among most men with low-grade (or low-risk) disease.24, 25 Thus, for men with low-grade disease, we recommend conservative therapy, incorporating observation within an active surveillance program. Second, for men with high-risk disease, early initiation of multi-modality therapy is recommended; 26,27,28 thus, these men are referred to our multi-disciplinary prostate cancer clinic. The screening algorithm was implemented as a low-cost and scalable clinical decision support tool into the system-wide electronic health record (EHR; Epic). The overarching goals of the development and implementation of a population-specific, risk-stratified prostate cancer screening algorithm were (1) to standardize the Duke Health network-wide clinical practice; (2) to identify individuals at high risk for aggressive prostate cancer; (3) and to avoid overscreening men at low risk. The primary aim of this study was to conduct a preliminary analysis of the impact of an EHR-based clinical decision support tool integrating this algorithm. In future analyses, with greater duration of follow-up, we will evaluate over- and underscreening, referral patterns, patient management, downstream costs, and outcomes.
METHODS
Setting and Patient Population
DPC is a large primary care network consisting of almost 300 clinicians (physicians and advanced practice providers) located in 40 clinic sites (26 sites providing continuity care for adults, the remainder provide urgent or pediatric care) that are predominantly community-based and spread across seven counties in north central North Carolina. The DPC serves nearly 300,000 unique patients with over 700,000 patient visits per year.
We evaluated the impact of implementing the risk-stratified prostate cancer screening algorithm on screening rates among men seen by a Duke primary care provider (PCP) in one of the 26 continuity clinics. All men aged 40–75 who were seen by a PCP between 02/01/2016 and 02/21/2018 were included in the analysis. We used a pre-post implementation study design, comparing men seen by a PCP from 2/1/2016–2/1/2017 (pre-implementation) to 2/2/2017–2/21/2018 (post-implementation). Note, these intervals were not equal: the post-implementation data was extracted on 2/22/2018 so the time frame includes visits until that date.
This study was reviewed and deemed exempt by the Duke Institutional Review Board..
Development and Implementation of the Duke Risk-Stratified Prostate Cancer Screening Algorithm
As noted above, the algorithm was developed by a multi-disciplinary group and intended to be used system-wide. The group’s goal was to provide clinicians with a standardized approach to prostate cancer screening that incorporated aspects of the USPSTF,15 ACS,18 and NCCN19 guidelines and acknowledged the shift in prostate cancer stage nationally. In particular, our algorithm included attention to high-risk individuals and risk stratification based on a midlife, baseline PSA.21 The algorithm and the importance of shared decision-making with patients were communicated by this multi-disciplinary group to primary care providers through various forums, including provider meetings at practices, practice medical director leadership meetings, and network-wide communication via email. We included several factors in the algorithm (Fig. 1), including age at time of examination, race (African American, other), and family history (first-degree relative with a history of prostate cancer prior to age 65; yes/no). We also incorporated the baseline and follow-up PSA levels in determining future monitoring intervals or referrals.
Implementation of Algorithm in EHR
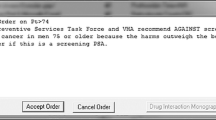
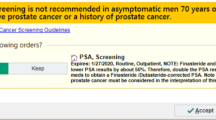
The algorithm was integrated as a clinical decision support tool into two components in the EHR. First, it was built within Health Maintenance, a task list that automatically populates and updates based on data in the EHR. This is an EHR section that is consistently utilized by PCPs to ensure patients are up-to-date on routine preventive and health maintenance care (Supplemental Figure 1). The Health Maintenance task list prompts a personalized “PSA Screening” reminder as per the algorithm. If the patient did not want to have PSA testing or if screening was not indicated (i.e., life expectancy < 10 years), then the clinician could record this in the Health Maintenance in the “Address Topic” field via drop down choices (i.e., “patient declines,” “not indicated”). However, this data is only captured as a snapshot in time in the EHR so it is not amenable for pre-post change analysis. Furthermore, the “Address Topic” field did not have to be filled and the informed decision documentation that takes place in a visit note is not captured by our analysis. Second, the algorithm age-based PSA cutoffs were built into the PSA laboratory results (Supplemental Figures 2–4). The age-specific criteria were then paired with a care recommendation to the ordering clinician to help determine next steps. The clinical decision support tool was not activated for patients with a history of prostate cancer. An example of the EHR logic is provided in Supplemental Figure 5.
Outcomes
Since we were interested in the uptake of the algorithm via the clinical decision support tool from 1 year pre- to 1 year post-implementation, we evaluated both the percent (and number) of men who met screening algorithm criteria and the percent (and number) of men who had a PSA. Men who met screening algorithm criteria were defined as having a PSA value, based upon age, race, family history, and the previous PSA level (if present), on record within the 27 months prior to the date of their PCP appointment. The same definition was used for pre- and post-implementation. The 27-month window was decided upon instead of 24 months to take into account variability in patient and PCP schedules.
Analytic Approach
Descriptive statistics comparing patient characteristics and differences in the proportion of men who met screening algorithm criteria in pre- and post-implementation time periods were performed followed by a one-sample pre-post test for binomial proportion and a two-proportion z test. Logistic repeated measures mixed models, which account for correlations due to repeated measures within individuals and correlations among individuals within sites, were used to test for differences in the proportion of individuals that met screening criteria in the pre- and post-implementation study periods. Time was included as a fixed effect (i.e., pre- vs. post-implementation). Clinic and subject within clinic were included as random effects, and age and race were included as covariates. Two models were implemented: (a) main effects model, age as a categorical variable (40–49, 50–69, 70–75) and (b) interaction model, interactions of time × race and time × age with age as a categorical variable (40–49, 50–69, 70–75). Race categories were summarized as Caucasian/White, Black or African American, Asian, Other, and not reported/declined/unavailable, with Caucasian/White included as the reference group. Models were performed using the glmer function of the lme4 package29 in R statistical software version 3.5.0.30 Fixed effects results are presented as odds ratio (95% confidence interval) and random effects are presented as variance/standard deviations. Confidence intervals were found using the confint function of the lme4 package based on the Wald method. To test for the overall effect of the categorical race, age, time × race, and time × age variables, likelihood ratio tests were performed.
Since the algorithm incorporated a discussion of screening among men aged 40–49, a more detailed evaluation regarding outcomes was performed in this age group. A retrospective chart review was conducted to determine the number of referrals to specialists, the number of men who had a prostate biopsy, and the number of cancers diagnosed for all men, pre- and post-implementation, aged 40–49, with a PSA level of 1.5 ng/ml or higher.21, 22
RESULTS
The study involved 49,053 and 49,980 men in the pre- and post-implementation periods, respectively, with 37,893 men having a PCP clinic visit in both periods (Table 1). Of the overall cohort, 20.6% of the men were African American.
Overall Findings
The implementation of the clinical decision support tool resulted in an increase in the percent of men who met screening algorithm criteria, from 49.3% pre-implementation to 68.0% post-implementation (p < 0.001) (Table 2). This increase was observed across all races, age categories, and PCP clinics.
Importantly, the percent of men who had a PSA did not change substantively: 55.3% pre-implementation and 55.0% post-implementation (Table 2). The percent of men aged 40–44 and 45–49 who had a PSA increased from pre- to post-implementation: 22.3% and 11.8%, respectively (Table 2). In contrast, for men aged 50–59, 60–69, and 70–75, the percent of men who had a PSA decreased by 8.0%, 8.4%, and 5.6%, respectively.
Mixed Models Results
Results of repeated measures mixed models, including age, race, and random effects of the clinic and subjects within a clinic without and with interactions, are provided in Table 3. Since the interactions were significant (time period × race and time period × age), the estimates we provide below are from the model with interactions. Overall, results suggest that the odds of meeting screening algorithm criteria was 6.5-times higher in the post-implementation period than in the pre-implementation period (95% CI, 5.97 to 7.05). Notably, the odds of meeting screening algorithm criteria or having a PSA completed in the post-implementation period was lower for men aged 50–69 and 70–75 (0.43 and 0.45, respectively). This was likely due to a decrease in PSA test ordering in the older age groups in the post-implementation period due to overscreening in the pre-implementation time frame. African American men were 1.57-times more likely to meet algorithm-based screening than Caucasian men (95% CI, 1.47 to 1.69). In contrast, Asian men were 21% less likely than Caucasian men to meet screening algorithm criteria (OR = 0.79, 95% CI, 0.68 to 0.91). The variance of the random clinic effect was 0.39 and the variance of the subject within clinic effect was 2.55. The intraclass correlation coefficient (ICC) of generalized linear models varies according to the values of the covariates and is less interpretable than the median odds ratio (MOR).31 Therefore, to better understand random effect variation, we present the median odds ratio. The MOR from the multi-level mixed model was 1.81 whereas ORs for patient-level characteristics (i.e., age) were of greater magnitude suggesting that unexplained between-clinic variation was not as relevant as patient-level characteristics for understanding rates of meeting screening criteria.
Subanalysis of Men Aged 40–49 Years
About 3% and 5% of men aged 40–49 had a PSA > 1.5 ng/ml in the pre-implementation and post-implementation period, respectively (Table 4). Of those men, about a quarter were aged 40–44 in either the pre-implementation (22.7%) or post-implementation (23.5%) and about three-quarters were aged 45–49 in either the pre-implementation (77.3%) or post-implementation (73.2%). Whereas PCPs repeated the PSA in the pre-implementation period in only 6.3% of these men, they repeated the PSA in 28.4% of the men in the post-implementation period. Of note, while the number of men who were referred to Duke Health’s urology service increased, the percent of referred men who had a biopsy decreased from 31.6 to 10.2%. The percent of men with prostate cancer on biopsy stayed about the same (27.2% pre-implementation; 33.3% post-implementation). In the pre- and post-implementation period, three and eleven men, respectively, were diagnosed with prostate cancer.
DISCUSSION
To our knowledge, this is the first study to evaluate the impact of an EHR-based clinical decision support tool incorporating a population-specific, risk-stratified prostate cancer screening algorithm. This low-cost, easily adaptable approach resulted in an increase in the percent of men seen by PCPs at Duke Primary Care who met screening algorithm criteria with a concurrent reduction in inappropriate screening. This change was observed across all primary care clinics. Moreover, while the percent of men who met screening algorithm criteria increased by almost 20%, from 49 to 68%, the percent of men who had documented PSA testing did not materially change. Rather, we observed a reduction in practice variation and a decrease in the rate of annual PSAs ordered by PCPs.
Historically, changing clinician practice is challenging. A classic example is the lengthy interval from evidence of the benefits of beta blockers following a myocardial infarction to implementation in clinical practice.32,33,34 Guideline dissemination methods have varying effectiveness and many lead to only modest or even no impact on changing practice.35,36,37,38 While incorporation in the EHR has been considered a potential implementation strategy, findings from studies have been mixed.37, 39,40,41,42 The Duke Health simple implementation strategy using Health Maintenance combined with tailored laboratory results with follow-up recommendations resulted in substantially improved algorithm-based screening while reducing practice variation. Utilizing the EHR and seamlessly integrating it into the workflow of PCPs offer a generalizable and scalable implementation strategy that can be applied to other topics and in other settings.
The debate regarding prostate cancer screening has often vascillated between doing more versus less. The Duke Health multi-disciplinary group’s goal was to improve appropriate screening recognizing an important at-risk population in the community, African American men. After implementation, the number of men who met screening algorithm criteria increased, regardless of age, race, or clinic. This change occurred without driving more testing as the volume of men with PSAs stayed constant. This was due to reduced testing among men in the older age groups and increased screening among men aged 40–49 in the post-implementation period.
As noted earlier, national guidelines have differed in whether to discuss prostate cancer screening among men aged 40–49. Based upon the findings of Vickers, Preston, and others showing the benefit of a baseline PSA for men in their forties,21, 22, 43 the multi-disciplinary group at Duke Health opted to start the discussion sometime between the ages of 40 and 49 years. This likely resulted in an increase in cost of care for this age group, as the number of men who had a test, were referred to a specialist for an elevated PSA, and had a biopsy increased. Whether the number and the aggressiveness of prostate cancer among men in this age group will offset the cost of care cannot be ascertained at this point. Currently, several men are still undergoing evaluation; it will require a longer interval of follow-up and a detailed cost analysis to better assess the impact of the algorithm on this age group. Nevertheless, after this preliminary evaluation and as part of our Learning Health System,44, 45 the multi-disciplinary group decided to change the system-wide recommendation to initiating the discussion between the ages of 45 and 49 rather than between 40 and 49.
While this study has several strengths, including a system-wide evaluation of a simple EHR implementation strategy among a large number of men from diverse backgrounds, there are several limitations that need to be considered when interpreting the findings. There is no broad consensus on PSA screening so the algorithm itself is without clinical and trial data that directly supports it. Additionally, evidence supporting broader screening for high-risk and African American men is limited which is why USPSTF does not make specific recommendations for these groups3, 16. This was a single-health care system implementation; implementation may be more difficult in a non-integrated system without a shared EHR. This was a prospective quality improvement project that was not randomized and with a time frame that was too short to evaluate the impact of the algorithm on prostate cancer detection, particularly among African American men. The pre- post-design and short time frame also limit delineation of temporal trends and factors that can impact screening rates. Future efforts will be able to better capture outcomes and costs associated with the algorithm implementation. Through data extraction from the EHR, we were not able to easily capture the cases in which the PCP discussed screening and the patient declined to have PSA screening; thus, we likely underestimated the percent of men meeting algorithm-based screening and do not have much insight into patient preferences around screening. Lastly, family history is not easily and reliably captured in the EHR as it is a hand-entered variable.
In conclusion, an EHR-based clinical decision support tool led to a significant increase in PCPs following the Duke Health system-wide PSA screening algorithm while avoiding an increase in number of men with a PSA. In other words, this preliminary analysis demonstrates that the Duke Health multi-disciplinary group may meet the goal of screening those who might be most likely to benefit and reducing screening in those who are unlikely to benefit. Our study highlights a potential pathway to leverage the EHR to influence and standardize PCP practice through better guideline adherence and reduced practice variation.
References
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER cancer statistics review, 1975-2016. National Cancer Institute. Bethesda, April 2019. https://seer.cancer.gov/csr/1975_2016/.
Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7-34, 2019
Schroder FH, Hugosson J, Roobol MJ, et al: Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 384:2027-35, 2014
Fenton JJ, Weyrich MS, Durbin S, et al: Prostate-Specific Antigen-Based Screening for Prostate Cancer: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 319:1914-1931, 2018
Gomella LG, Liu XS, Trabulsi EJ, et al: Screening for prostate cancer: the current evidence and guidelines controversy. Can J Urol 18:5875-83, 2011
Burns RB, Olumi AF, Owens DK, et al: Would You Recommend Prostate-Specific Antigen Screening for This Patient?: Grand Rounds Discussion From Beth Israel Deaconess Medical Center. Ann Intern Med 170:770-778, 2019
Welch HG, Black WC: Overdiagnosis in cancer. J Natl Cancer Inst 102:605-13, 2010
Moyer VA, Force USPST: Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157:120-34, 2012
Smith RA, Manassaram-Baptiste D, Brooks D, et al: Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 64:30-51, 2014
Mohler JL, Kantoff PW, Armstrong AJ, et al: Prostate cancer, version 2.2014. J Natl Compr Cancer Netw 12:686-718, 2014
Jemal A, Fedewa SA, Ma J, et al: Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA 314:2054-61, 2015
Houston KA, King J, Li J, et al: Trends in Prostate Cancer Incidence Rates and Prevalence of Prostate Specific Antigen Screening by Socioeconomic Status and Regions in the United States, 2004 to 2013. J Urol 199:676-682, 2018
Pinsky PF, Prorok PC, Kramer BS: Prostate Cancer Screening - A Perspective on the Current State of the Evidence. N Engl J Med 376:1285-1289, 2017
Pinsky PF, Prorok PC, Yu K, et al: Extended mortality results for prostate cancer screening in the PLCO trial with median follow-up of 15 years. Cancer 123:592-599, 2017
Force USPST, Grossman DC, Curry SJ, et al: Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 319:1901-1913, 2018
Andriole GL, Crawford ED, Grubb RL, 3rd, et al: Mortality results from a randomized prostate-cancer screening trial. N Engl J Med 360:1310-9, 2009
DeSantis CE, Miller KD, Goding Sauer A, et al: Cancer statistics for African Americans, 2019. CA Cancer J Clin 69:211-233, 2019
Smith RA, Andrews KS, Brooks D, et al: Cancer screening in the United States, 2018: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 68:297-316, 2018
Mohler JL, Antonarakis ES, Armstrong AJ, et al: Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 17:479-505, 2019
Vickers AJ, Cronin AM, Bjork T, et al: Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ 341:c4521, 2010
Vickers AJ, Ulmert D, Sjoberg DD, et al: Strategy for detection of prostate cancer based on relation between prostate specific antigen at age 40-55 and long term risk of metastasis: case-control study. BMJ 346:f2023, 2013
Preston MA, Batista JL, Wilson KM, et al: Baseline Prostate-Specific Antigen Levels in Midlife Predict Lethal Prostate Cancer. J Clin Oncol 34:2705-11, 2016
Aminsharifi A, Schulman A, Anderson J, et al: Primary care perspective and implementation of a multidisciplinary, institutional prostate cancer screening algorithm embedded in the electronic health record. Urol Oncol 36:502.e1-502.e6, 2018
Wilt TJ, Brawer MK, Jones KM, et al: Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 367:203-13, 2012
Wilt TJ, Jones KM, Barry MJ, et al: Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N Engl J Med 377:132-142, 2017
Parker CC, James ND, Brawley CD, et al: Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392:2353-2366, 2018
Sydes MR, Spears MR, Mason MD, et al: Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol 29:1235-1248, 2018
Bill-Axelson A, Holmberg L, Garmo H, et al: Radical Prostatectomy or Watchful Waiting in Prostate Cancer - 29-Year Follow-up. N Engl J Med 379:2319-2329, 2018
Bates D, Machler M, Bolker BM, et al: Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw 67:1-48, 2015
R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2018. https://www.R-project.org/
Merlo J, Chaix B, Ohlsson H, et al: A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 60:290-7, 2006
Krumholz HM, Radford MJ, Wang Y, et al: National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA 280:623-9, 1998
Sial SH, Malone M, Freeman JL, et al: Beta blocker use in the treatment of community hospital patients discharged after myocardial infarction. J Gen Intern Med 9:599-605, 1994
Ayanian JZ, Hauptman PJ, Guadagnoli E, et al: Knowledge and practices of generalist and specialist physicians regarding drug therapy for acute myocardial infarction. N Engl J Med 331:1136-42, 1994
Grol R, Grimshaw J: From best evidence to best practice: effective implementation of change in patients’ care. Lancet 362:1225-1230, 2003
Fischer F, Lange K, Klose K, Greiner W, Kraemer A: Barriers and strategies in guideline Implementation-A Scoping Review. Healthcare (Basel)4(3):36, 2016. https://doi.org/10.3390/healthcare4030036.
Dowding D, Randell R, Gardner P, et al: Dashboards for improving patient care: review of the literature. Int J Med Inform 84:87-100, 2015
Twohig PA, Rivington JR, Gunzler D, et al: Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res 19:475, 2019
Baron RC, Melillo S, Rimer BK, et al: Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers a systematic review of provider reminders. Am J Prev Med 38:110-7, 2010
Jun T, Kwang H, Mou E, et al: An Electronic Best Practice Alert Based on Choosing Wisely Guidelines Reduces Thrombophilia Testing in the Outpatient Setting. J Gen Intern Med 34:29-30, 2019
Ancker JS, Edwards A, Nosal S, et al: Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak 17:36, 2017
Brown B, Young J, Smith DP, et al: A multidisciplinary team-oriented intervention to increase guideline recommended care for high-risk prostate cancer: A stepped-wedge cluster randomised implementation trial. Implement Sci 13(1):43, 2016. https://doi.org/10.1186/s13012-018-0733-x.
Preston MA, Gerke T, Carlsson SV, et al: Baseline Prostate-specific Antigen Level in Midlife and Aggressive Prostate Cancer in Black Men. Eur Urol 75:399-407, 2019
Guise JM, Savitz LA, Friedman CP: Mind the Gap: Putting Evidence into Practice in the Era of Learning Health Systems. J Gen Intern Med 33:2237-2239, 2018
Nwaru BI, Friedman C, Halamka J, et al: Can learning health systems help organisations deliver personalised care? BMC Med 15:177, 2017
Funding
This work was supported by grants from the National Cancer Institute (P30CA014236) and the Duke Institute for Health Innovation (DIHI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was reviewed and deemed exempt by the Duke Institutional Review Board.
Conflict of Interest
Thomas J. Polascik reports consultancies in the last 3 years and honoraria in the last 3 years from Healthtronics [training agreement]. Terry Hyslop reports consultancies in the last 3 years from AbbVie. Glenn M. Preminger reports consultancies in the last 3 years from Boston Scientific, Auris Robotics, and Kalera Medical and reports other relationships as an Associate Editor for Up to Date. Kevin Shah reports stock ownership/options other than mutual funds from Infinity Pharmaceuticals. Anand Shah reports stock ownership/options other than mutual funds from Pfizer Inc. All other authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Previous Presentation
2019 Society of General Internal Medicine Annual Meeting
Rights and permissions
About this article
Cite this article
Shah, A., Polascik, T.J., George, D.J. et al. Implementation and Impact of a Risk-Stratified Prostate Cancer Screening Algorithm as a Clinical Decision Support Tool in a Primary Care Network. J GEN INTERN MED 36, 92–99 (2021). https://doi.org/10.1007/s11606-020-06124-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-020-06124-2