Abstract
Background
Despite numerous interventions targeting medication adherence in patients with uncontrolled hypertension, practice-based trials in Latino patients are scant.
Objective
To evaluate the effect of a systems-level adherence intervention, delivered by medical assistants (MAs), versus a comparison condition on medication adherence and blood pressure (BP) in 119 hypertensive Latino patients who were initially non-adherent to their antihypertensive medications.
Study Design
Randomized control trial.
Participants
Patients (50% women; mean age, 61 years) were recruited from April 2013 to August 2015 in a community-based practice in New York.
Intervention
Systems-level approach that included an office system component built into the electronic health record and a provider support component consisting of nine MA-delivered health coaching sessions for improving medication adherence. The comparison group received the standard health coaching procedures followed at the clinic.
Main Outcome Measures
The primary outcome was rate of medication adherence measured by an electronic monitoring device (EMD) across 6 months. The secondary outcomes were self-reported medication adherence measured by the eight-item Morisky Medication Adherence Scale (MMAS-8) and BP reduction from baseline to 6 months.
Key Results
Adherence as measure by EMD worsened for both groups (p = 0.04) with no between-group difference (− 9.6% intervention and − 6.6% control, p = 0.66). While systolic BP improved in both groups, the difference between groups was not significant (− 6 mmHg in intervention vs. − 2.7 mmHg in control, p = 0.34). In contrast, the intervention group had a greater improvement in self-reported adherence (mean change 1.98 vs. 1.26, p = 0.03) when measured using the MMAS-8.
Conclusions
Among Latinos with poorly controlled BP who were non-adherent to their antihypertensive medications, a systems-level intervention did not improve adherence as measured by EMD nor blood pressure. However, many patients reported challenges to using the EMD. Improvements in self-reported adherence suggest that this measure captures different aspects of adherence behavior than EMD.
Clinical Trial Registration
NCT03560596
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Latinos have the lowest blood pressure (BP) control rates in the USA (34% vs. 53% and 43% in white and black adults).1, 2 Data from the Hispanic Community Health Study/Study of Latinos showed significant deficits in the treatment and control of hypertension (HTN), specifically for Latinos of Central American, South American, and Dominican ancestry (control rates 19, 31, and 33%) compared with Mexican Americans (34%).3 In longitudinal studies, Latinos aged 45–74 years exhibited a higher incidence rate (IRR) of HTN as compared with whites (IRR = 1.29; 95% confidence interval [CI] 1.06–1.57).4 The combination of these factors (increasing incidence and poor control rates) is driving increases in HTN-related mortality at a faster rate in Latinos than in other ethnic groups.5
Medication non-adherence may explain the poor BP control in Latinos.6,7,8,9,10 Recent data showed that hypertensive Latino adults reported the lowest adherence to their medications (67%) compared with black and white adults (77% for both groups).11 Similarly, in the Health and Retirement Study, Latinos reported poorer adherence (53%) compared with white (64%) and black (73%) older adults.6 Despite many interventions designed to improve medication adherence in patients with uncontrolled HTN, practice-based randomized controlled trials (RCT) in Latino patients are scant. We are aware of only one study that targeted adherence in an exclusively Latino sample.12 In this study, brief counseling by a bilingual pharmacist and home BP monitoring significantly increased adherence among 53 Latinos with uncontrolled HTN.12 However, the study lacked a control group and did not standardize the intervention procedures. Thus, the development of interventions targeted at improving adherence in this high-risk population is urgently needed to address the racial/ethnic disparities in BP control.
In this paper, we report the results of Ayudando a Latinos Hipertensos Para Mejorar Adherencia a sus Medicamentos (ALMA), a pragmatic RCT that evaluated the effect of a systems-level intervention, delivered by medical assistants (MAs), on medication adherence and BP among Latino patients with uncontrolled HTN, who were initially non-adherent to their antihypertensive medications. We hypothesized that the intervention would result in a higher proportion of patients who are adherent to their antihypertensive medication, as assessed by an electronic monitoring device (EMD) at 6 months versus an enhanced comparison condition. The secondary aims tested whether the adherence intervention would result in better self-reported medication adherence and a greater reduction in BP at 6 months in the intervention than in the comparison group.
METHODS
Setting and Participants
As previously described,13 we recruited patients at a community-based clinic that serves predominantly Latino patients in New York City. The partnering clinic is a patient-centered medical home that has been nationally recognized for its labor innovations in which MAs are trained as health coaches.14 Prior to becoming a health coach, MAs undergo a 9-month training curriculum in chronic disease management, patient-centered communication skills, and principles of behavior change.
Eligible patients self-identified as Latino; were ≥ 18 years old; had uncontrolled HTN (i.e., SBP ≥ 140 mmHg or DBP ≥ 90 mmHg or SBP ≥ 130 mmHg or DBP ≥ 80 mmHg for patients with diabetes or chronic kidney disease on at least two visits in the past year); were taking at least one antihypertensive medication and were non-adherent to their antihypertensive medication; had at least one comorbid diagnosis (i.e., hyperlipidemia, diabetes, and/or kidney disease); and did not have an active serious mental illness (e.g., major depressive disorder, schizophrenia), as documented in the EHR and verified by the patient’s PCP.
Trained research assistants (RAs) who were native Spanish speakers conducted all recruitment and assessment visits, in the patient’s preferred language. The RAs used a web-based tool that allowed for direct data entry while using a conversational interview style. RAs recruited patients using three strategies: (1) PCP and MA referrals of potentially eligible patients; (2) calling patients from a list created from the clinic electronic health record (EHR) using the ICD-9 codes for HTN, after PCP approval; and (3) self-referral from posters hung in the waiting room.13 The study was approved by the NYU Institutional Review Board. The trial is registered at https://www.clinicaltrials.gov: NCT03560596Footnote 1.
One-month run-in period
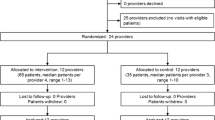
At the initial screening visit, RAs administered the consent form and survey measures (Table 1); after which, all potentially eligible patients began a 1-month run-in period to assess their adherence using the validated eight-item Morisky Medication Adherence Scale (MMAS-8).15,16,17 The first seven items require a yes/no response; the final item uses a 5-point scale (never/rarely to all the time). Total MMAS-8 scores range from 0 to 8; higher scores reflect better adherence.15 Only patients who were non-adherent to their medications at the conclusion of the 1-month period (MMAS-8 < 6)Footnote 2 were eligible for the study (Fig. 1).15
Randomization
After confirming their non-adherent status, eligible patients completed the baseline measures (Table 1) and were then randomized in a 1:1 ratio to the intervention or comparison group. Block randomization was used to ensure a roughly equal assignment of patients to the groups. The randomization group was kept in a secure electronic file that only restricted staff could access.18, 19 Upon randomization, the study coordinator sent an EHR alert to the MA responsible for delivering the intervention.
Intervention Condition
Details of the intervention are described elsewhere.13 Briefly, the intervention was culturally adapted during the formative phase of the trial based on iterative feedback from an Academic-Community Advisory Board, focus groups, and published literature on Latino treatment beliefs.20,21,22,23,24,25,26,27,28 The systems-level intervention was based on the evidence-based teamlet model that targeted improving implementation of the antihypertensive regimen (e.g., skipped, extra, or fewer doses of prescribed medications).29, 30 Teamlets are two-person teams, consisting of a PCP and MA who work consistently and collaboratively to care for a panel of patients.30 The intervention targeted both patients and providers, and was comprised of an office system component and a PCP support component. The office system component was built into the EHR to facilitate identification of Latino patients with uncontrolled HTN and their referral to a bilingual MA. The PCP support component consisted of MA-delivered health coaching for improving adherence. MAs who served as the study interventionists attended a 2-day training in the delivery of the intervention and motivational interviewing. Booster training was conducted throughout the study to prevent drift in MA counseling skills. Details of the training are reported elsewhere.13
During the sessions, MAs followed a semi-structured counseling script that was tailored to the multiple determinants of adherence (e.g., cultural, psychosocial, behavioral) that affect Latino patients’ medication-taking behaviors based on our formative phase.22 MAs followed the counseling script to explore patients’ barriers and facilitators to adherence, and to assist them in developing an action plan. The counseling script was embedded into the EHR to allow MAs the ability to systematically record patients’ responses and retrieve progress notes from previous sessions. PCPs could also view progress notes during clinic visits with intervention patients. Patients in the intervention group participated in nine 15-min counseling sessions conducted in person or via telephone over 6 months.
Enhanced Comparison Condition
The comparison group received the standard health coaching procedures followed at the clinic (e.g., providing general health education; conducting BP checks). We took several precautions to prevent the utilization of the counseling script with patients in the comparison group including (1) assigning only half of the MAs to deliver the intervention and (2) restricting access to the script for patients in the comparison group. Regardless of group assignment, patients received standard treatment as determined by their physicians.
Outcome Assessments
The primary outcome was the rate of medication adherence across the 6-month post-randomization period.13 Medication adherence was assessed with Ecaps (Information Mediary Corporation), a validated EMD that has demonstrated 99.6% event accuracy in clinical trials. Patients were given one EMD to monitor one of their antihypertensive medications. For each patient, we calculated rates of EMD-measured adherence as the percent of prescribed doses removed by the patient during the study monitoring period. We accounted for periods of “pocket dosing” (e.g., use of pillboxes) in the analyses.13, 31 Secondary outcomes included change in self-reported medication adherence and BP from baseline to 6 months. Patient self-reported adherence was assessed using the MMAS-8 (α = 0.83).15,16,17 BP was assessed using validated automated WatchBP monitors at all study visits, following the American Heart Association guidelines.13 The average of three BP readings was used as the measurement for each study visit.
Other Assessments
RAs abstracted clinical data from patients’ EHR at the initial screening visit and 6-month visit including the following: duration of HTN, total number and classes of antihypertensive medications, and comorbid conditions. Data on patient socio-demographic characteristics were also collected at the screening visit.
Statistical Analysis
Sample size was determined using a moderate change in adherence rates (0.20 between-group difference) as the effect size, power of 0.80 and significance level of α = 0.05.13 This analysis suggested a sample size of 74 patients per group. Due to recruitment challenges,10 we recruited a total of 119 patients for this study. We conducted a post hoc power analysis32 to determine our realized power, the hypothesized effect size used in our original calculations, and the actual sample size. This resulted in a statistical power of 0.70.
Fisher’s exact test was used to compare the groups on the categorical baseline variables while independent sample t tests were used for continuous variables. A repeated measures linear mixed effects regression model approach was used to account for continuous adherence outcomes measured at the follow-up visits. An unstructured variance covariance matrix was used to estimate the error terms to test the parameters from the regression models. The values of continuous outcomes were modeled as functions of time, treatment, and the interaction of time and treatment. Our analytic strategy handles missing data by estimating model parameters for each individual based on that individual’s available data (full information maximum likelihood).
We tested the secondary aims in a similar manner to the primary aim. Self-reported adherence was assessed as a continuous outcome using a repeated measures linear mixed effects regression model. SBP and DBP were treated as continuous variables, and the effect of treatment was assessed using linear mixed effects regression models.
RESULTS
From April 2013 to August 2015, we screened 221 patients for eligibility during the 1-month run-in period. Of these patients, we excluded 102 (46.1%) because they either were adherent to their antihypertensive medications (n = 88) or did not complete the visit (n = 14). Thus, we randomized 119 patients (53.4%) into the trial; 60 patients were allocated to the intervention group and 59 patients to the comparison group (Fig. 2). Of the 119 patients, 102 (86%) completed the 6-month visit: 50 (83%) from the intervention group and 52 (88%) from the comparison group. There were no significant differences between patients who completed the trial and those lost to follow-up.
The mean age was 60.8 years (standard deviation [SD] 11.2); 50% were women; 38% had annual household income < $20,000, 59% had less than a high school degree, and 64% primarily spoke Spanish (Table 2). Mean baseline BP was 141.8 (SD 16.3)/81.2 (SD 10.8) mmHg. There were no significant differences between the groups on the baseline measures.
Medication Adherence Assessed by EMD (Primary Outcome)
Of the 119 EMDs distributed to patients, analyzable data were available on 94 (80%) patients. Thirty-eight (32%) patients had data at all three time points. Twenty-five patients (20%) had no EMD data (8 [32%] patients from the intervention group and 17 [68%] from the comparison group; p = 0.21). Reasons for missing EMD data included the following: the patient did not use the EMD or was lost to follow-up (n = 10 [8%]), the EMD malfunctioned (n = 5 [4%]), and the patient lost the EMD (n = 10 [8%]). Females were 2.5 times more likely to have no EMD data than males (chi-square = 3.91, p = 0.05, 95% CI 1.01–2.5).
EMD-measured adherence at baseline (i.e., after the 1-month period) was similar for both groups (84.1% (SD 26.8%) for the intervention group vs. 79% (SD: 23.6%) for the comparison group, p = 0.34). Of the 94 patients with EMD data, adherence rates at 6 months were 74.5% (SD 23.5%) for the intervention group versus 72.4% (SD 22.2%) for the comparison group. There was a significant reduction in adherence rates across the 6-month study for both arms (Table 3). Due to the decline in adherence over time, which was in the opposite direction of what was hypothesized, we did not complete sensitivity analysis.
Secondary Outcomes
Adherence Assessed by Self-report
The mean self-reported adherence for the intervention and comparison groups at baseline was 4.32 (SD 1.41) and 4.65 (SD 1.14; range 0–8). At 6 months, the intervention group had a significantly greater improvement in adherence than the comparison group (mean change 1.98 versus 1.26, p = 0.03; Tables 3 and 4).
Change in BP
Systolic BP significantly improved in both groups across the 6 months (p = 0.02). The intervention group showed a 3.3 mmHg greater reduction in SBP than the comparison group by 6 months, with no between-group difference (p = 0.34). The intervention group showed a 2.8 mmHg greater reduction in DBP than the comparison group, with no between-group difference (p = 0.18).
DISCUSSION
In this practice-based trial, we evaluated the effect of a systems-level intervention, delivered by trained MAs, on medication adherence and BP among hypertensive Latinos who were initially non-adherent to their antihypertensive medications. Contrary to our hypotheses, we did not observe a significant between-group difference in our primary outcome of EMD-measured adherence and secondary outcome of BP reduction. We observed a decline in EMD-measured adherence over the 6-month trial in both groups. The decrease in EMD-measured adherence in our study is consistent with research showing that adherence levels are inflated at the start of EMD monitoring due to the combined novelty of the device and patients’ awareness of being monitored (e.g., Hawthorne effect).33,34,35,36 Similar to our findings, studies have demonstrated distinct decreases in adherence over time with longer EMD monitoring periods (i.e., ≥ 6 months).37, 38 Based on this data and the barriers to patient recruitment associated with using EMD, we used the MMAS-8 as our screening tool to determine patient eligibility.13 Thus, patients may have entered the study if they self-reported non-adherent behaviors and their EMD-measured adherence was > 80%.
Our study follows best practices to use a multi-measure approach (i.e., adherence measured by EMD and self-report) to increase the accuracy, feasibility, and practicality of medication adherence assessment.39, 40 As previously discussed,13 we experienced several challenges with the EMDs in this patient population, including patient difficulty in understanding how to use the device and mistrust of the device, which greatly inhibited patient use of the device. However, we found a positive impact of the intervention on our secondary outcome of self-reported adherence measured with the MMAS-8. The significant effect of our intervention on self-reported adherence is comparable with the study by Thom et al., which found that minority patients (70% Latino) randomized to receive MA-led health coaching reported higher adherence to their cardiovascular medications than those in usual care.41 However, in the Thom et al. study, adherence was assessed with a single-item self-report measure and did not include EMD-measured adherence.41
While it may seem counterintuitive that our intervention would result in a significant decrease in EMD-measured adherence and increase in self-reported adherence, several other studies have documented this phenomenon when using both types of adherence assessments.42,43,44 While the measures produce low levels of agreement, in actuality, EMDs and self-report measures accurately depict different aspects of patients’ adherence behaviors.43, 44 EMDs, which are considered the “gold standard” of adherence measurement, capture a temporal history of each medication dose. Alternatively, validated self-report measures such as the MMAS-8 give information on the patient’s perception of how often they take their medications, and capture the reasons why patients do not adhere. Thus, while our intervention may have positively impacted the reasons why patients may be non-adherent to their antihypertensive medications, it did not impact the percentage of prescribed doses taken, as measured by EMD nor BP.
There are several strengths of our study. First, our counseling script was embedded within the EHR to facilitate the integration of the intervention into the workflow. Second, our study targeted hypertensive patients who were non-adherent to their medications, thereby enrolling high-risk patients that are more likely to be high users of the healthcare system. Third, as opposed to previous health coaching trials,41, 45 we developed our culturally tailored intervention with input from hypertensive Latino patients to specially address non-adherence to antihypertensive medications. Last, to our knowledge, this is the first RCT focusing on improving adherence and BP in a diverse sample of Spanish-speaking Latinos, providing important information beyond cardiovascular risk in Mexican Americans.
Despite these strengths, this study produced null findings. Reasons may include the following: Our primary outcome analysis may have been underpowered to detect differences due to the high proportion of missing EMD data. While our intervention group exhibited a 3.3 mmHg greater reduction in SBP than the comparison group at 6 months, our study was not powered to detect such small differences. EMDs may also not be the best way to capture adherence data in this population, as only 32% of patients had EMD data at all time points. Interviews conducted at the end of the trial showed that many patients chose not to use the EMDs for various reasons, despite multiple attempts by the study team to increase their comfort and familiarity with the device. Since all clinic patients receive MA-delivered health education and BP monitoring as standard practice, patients in the comparison group were likely exposed to an “intervention effect” limiting our ability to detect differences. Cost constraints allowed for monitoring patients’ primary antihypertensive medication. While this does not reflect adherence to other medications, evidence shows that patterns of adherence to one medication often reflect adherence to others.46 Finally, while medication adherence is a primary contributor to BP control, other factors such as changes in lifestyle behaviors are also important for BP control but were not measured in this study.
In conclusion, our systems-level intervention did not result in improvements in our primary outcome of EMD-measured adherence. We also did not observe significant group differences in BP reduction. However, we found that our intervention was associated with significant improvements in self-reported adherence among high-risk Latinos with uncontrolled HTN.
Notes
Due to administrative errors in the clinicaltrials.gov account, the date of record indicates that the trial was registered after the completion of the trial. The original record was created July 17, 2012, and within 2 weeks of receipt of the original funding notice.
Note that this scoring is the reverse of what was published in the protocol paper and reflects the corrected scoring as published by Moriksy and outlined in the license agreement. Reverse scoring the measure had no effect on the study sample.
References
Centers for Disease Control and Prevention. Racial/Ethnic disparities in the awareness, treatment, and control of hypertension - United States, 2003-2010. MMWR Morb Mortal Wkly Rep. 2013;62(18):351-355.
Gu A, Yue Y, Desai RP, Argulian E. Racial and Ethnic Differences in Antihypertensive Medication Use and Blood Pressure Control Among US Adults With Hypertension: The National Health and Nutrition Examination Survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes. 2017;10(1).
Sorlie PD, Allison MA, Aviles-Santa ML, et al. Prevalence of Hypertension, Awareness, Treatment, and Control in the Hispanic Community Health Study/Study of Latinos. Am J Hypertens. 2014; 27:793-800.
Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension (Dallas, Tex : 1979). 2011;57(6):1101-1107.
Guzman NJ. Epidemiology and management of hypertension in the Hispanic population: a review of the available literature. Am J Cardiovasc Drugs 2012;12(3):165-178.
Sudano JJ, Jr., Baker DW. Antihypertensive medication use in Hispanic adults: a comparison with black adults and white adults. Med Care 2001;39(6):575-587.
Raebel MA, Ellis JL, Carroll NM, et al. Characteristics of patients with primary non-adherence to medications for hypertension, diabetes, and lipid disorders. J Gen Intern Med 2012;27(1):57-64.
Ishisaka DY, Jukes T, Romanelli RJ, Wong KS, Schiro TA. Disparities in adherence to and persistence with antihypertensive regimens: an exploratory analysis from a community-based provider network. J Am Soc Hypertens 2012;6(3):201-209.
Rolnick SJ, Pawloski PA, Hedblom BD, Asche SE, Bruzek RJ. Patient characteristics associated with medication adherence. Clin Med Res. 2013 https://doi.org/10.3121/cmr.2013.1113.
Traylor AH, Schmittdiel JA, Uratsu CS, Mangione CM, Subramanian U. Adherence to cardiovascular disease medications: does patient-provider race/ethnicity and language concordance matter? J Gen Intern Med 2010;25(11):1172-1177.
Natarajan S, Santa Ana EJ, Liao Y, Lipsitz SR, McGee DL. Effect of treatment and adherence on ethnic differences in blood pressure control among adults with hypertension. Ann Epidemiol 2009;19(3):172-179.
Lai LL. Community pharmacy-based hypertension disease-management program in a Latino/Hispanic-American population. Consult Pharm 2007;22(5):411-416.
Schoenthaler A, De La Calle F, Barrios-Barrios M, et al. A practice-based randomized controlled trial to improve medication adherence among Latinos with hypertension: study protocol for a randomized controlled trial. Trials. 2015;16(1):290.
Nelson K, Pitaro M, Tzellas A, Lum A. Practice profile. Transforming the role of medical assistants in chronic disease management. Health Aff (Millwood) 2010;29(5):963-965.
Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol 2011;64(3):255-257; discussion 258-263.
Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10(5):348-354.
Berlowitz DR, Pajewski NM, Kazis LE. Intensive Blood-Pressure Treatment and Patient-Reported Outcomes. N Engl J Med 2017;377(21):2097.
Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol 2004;23(5):443-451.
Resnick B, Bellg AJ, Borrelli B, et al. Examples of implementation and evaluation of treatment fidelity in the BCC studies: where we are and where we need to go. Ann Behav Med 2005;29 Suppl:46-54.
Meyer D, Leventhal H, Gutmann M. Common-sense models of illness: the example of hypertension. Health Psychol 1985;4(2):115-135.
Baumann LJ, Leventhal H. “I can tell when my blood pressure is up, can’t I?”. Health Psychol 1985;4(3):203-218.
Leventhal H. The role of theory in the study of adherence to treatment and doctor-patient interactions. Med Care 1985;23(5):556-563.
Leventhal H, Cameron LD. Behavioral theories and the problem of compliance. Patient Educ Couns 1987;10:117-138.
Mann DM, Ponieman D, Leventhal H, Halm EA. Predictors of adherence to diabetes medications: the role of disease and medication beliefs. J Behav Med 2009;32(3):278-284.
Brown SA, Becker HA, Garcia AA, Barton SA, Hanis CL. Measuring health beliefs in Spanish-speaking Mexican Americans with type 2 diabetes: adapting an existing instrument. Res Nurs Health 2002;25(2):145-158.
van Servellen G, Nyamathi A, Carpio F, et al. Effects of a treatment adherence enhancement program on health literacy, patient-provider relationships, and adherence to HAART among low-income HIV-positive Spanish-speaking Latinos. AIDS Patient Care STDs 2005;19(11):745-759.
Ailinger RL, Martyn D, Lasus H, Lima Garcia N. The effect of a cultural intervention on adherence to latent tuberculosis infection therapy in Latino immigrants. Public Health Nurs 2010;27(2):115-120.
Kronish I, Leventhal H, Horowitz CR. Understanding Minority Patients’ Beliefs About Hypertension to Reduce Gaps in Communication Between Patients and Clinicians. J Clin Hypertens 2012;14:38-44.
Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114-1117.
Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med 2007;5(5):457-461.
Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008;21(10):1137-1143.
Cohen J.Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Erlbaum; 1988.
Deschamps AE, Van Wijngaerden E, Denhaerynck K, De Geest S, Vandamme A-M. Use of electronic monitoring induces a 40-day intervention effect in HIV patients. J Acquir Immune Defic Syndr 2006;43(2):247-248.
Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 1999;21(6):1074-1090; discussion 1073.
Wetzels GE, Nelemans PJ, Schouten JS, van Wijk BL, Prins MH. All that glisters is not gold: a comparison of electronic monitoring versus filled prescriptions--an observational study. BMC Health Serv Res 2006;6:8.
Wetzels GE, Nelemans P, Schouten JS, Prins MH. Facts and fiction of poor compliance as a cause of inadequate blood pressure control: a systematic review. J Hypertens 2004;22(10):1849-1855.
Waeber B, Leonetti G, Kolloch R, McInnes GT. Compliance with aspirin or placebo in the Hypertension Optimal Treatment (HOT) study. J Hypertens 1999;17(7):1041-1045.
Knafl GJ, Schoenthaler A, Ogedegbe G. Secondary analysis of electronically monitored medication adherence data for a cohort of hypertensive African-Americans. Patient Prefer Adherence 2012;6:207-219.
Lam WY, Fresco P. Medication Adherence Measures: An Overview. Biomed Res Int 2015;2015:217047-217047.
Kreys E. Measurements of Medication Adherence: In Search of a Gold Standard. J Clin Pathways 2016;2:43-47.
Thom DH, Willard-Grace R, Hessler D, et al. The impact of health coaching on medication adherence in patients with poorly controlled diabetes, hypertension, and/or hyperlipidemia: a randomized controlled trial. J Am Board Fam Med 2015;28(1):38-45.
Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care 2004;42(7):649-652.
Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028-3035.
DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42(3):200-209.
Margolius D, Bodenheimer T, Bennett H, et al. Health coaching to improve hypertension treatment in a low-income, minority population. Ann Fam Med 2012;10(3):199-205.
Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med 1990;150(9):1881-1884.
Acknowledgments
The authors would like to thank the Health Coaches (Palmira Brown, Cindy Ruiz, Ana Ventura, Jacqueline Camacho, and Luis Carrasco) at Union Health Center for their dedication to this project in the delivery of the intervention. Morisky Widget™, MMAS™, MMAS4™, MMAS-8™, Morisky Medication Adherence Protocol™, and Morisky Medication Adherence Scale™ content, name, and trademarks are protected by U.S. and International Trademark and Copyright laws. Permission for use of the scale and its coding is required. A license agreement is available from Donald E. Morisky, ScD, ScM, MSPH, 14735 NE 20th St Bellevue, WA 98007, USA; dmorisky@gmail.com or Trubow1@gmail.com.
Funding
This work was financially supported by grant 12GRNT11670001 from the American Heart Association (PI: Schoenthaler).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the NYU Institutional Review Board.
Conflict of Interest
The authors declare that they do not have a conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schoenthaler, A., de la Calle, F., Pitaro, M. et al. A Systems-Level Approach to Improving Medication Adherence in Hypertensive Latinos: a Randomized Control Trial. J GEN INTERN MED 35, 182–189 (2020). https://doi.org/10.1007/s11606-019-05419-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-019-05419-3