ABSTRACT
Statins are some of the most widely prescribed medications, and though generally well tolerated, can lead to a self-limited myopathy in a minority of patients. Recently, these medications have been associated with a necrotizing autoimmune myopathy (NAM). Statin-associated NAM is characterized by irritable myopathy on electromyography (EMG) and muscle necrosis with minimal inflammation on muscle biopsy. The case presented is a 63-year-old woman who has continued elevation of creatine kinase (CK) after discontinuation of statin therapy. She has irritable myopathy on EMG and NAM is confirmed by muscle biopsy. She subsequently tests positive for an experimental anti-3-hydroxy-3-methylglutaryl-coenzyme A (anti-HMGCoA) antibody that is found to be present in patients with statin-associated NAM. Though statin-associated NAM is a relatively rare entity, it is an important consideration for the general internist in patients who continue to have CK elevation and weakness after discontinuation of statin therapy. Continued research is necessary to better define statin-specific and dose-dependent risk, as well as optimal treatment for this condition.
Similar content being viewed by others
CASE PRESENTATION
A 63-year-old African American woman with a past medical history of mild intermittent asthma, allergies, coronary artery disease, gastroesophageal reflux, hypertension and type 2 diabetes mellitus presented for evaluation of proximal muscle weakness most prominent in the lower extremities. She complained that she was unable to walk without her legs buckling. She denied excessive alcohol use, weight loss, night sweats, black tarry stools, joint swelling, fevers, and skin changes. Examination confirmed significant proximal muscle weakness of her lower extremities. Routine laboratory studies were normal except for a creatine kinase (CK) level of 10,829 U/L (Fig. 1). Several months prior to the onset of her weakness, she underwent coronary artery stenting and was changed from simvastatin to atorvastatin. Before switching to atorvastatin, her CK level was normal (174 U/L). The atorvastatin was suspected as the cause and immediately discontinued. After several months of taking no statins, her walking improved, but she continued to have significant muscle pain and weakness. Her CK was rechecked and remained elevated at 2306 U/L (Fig. 1).
Additional neurologic, serologic, and musculoskeletal studies were performed. Nerve conduction study (NCS) and electromyography (EMG) were consistent with an active, irritable myopathy of the proximal muscles. Myositis-associated and connective tissue disease antibody panels were negative except for positive titers of anti-centromere antibodies (Table 1). A muscle biopsy was performed that showed muscle necrosis with minimal inflammation. At that time, no definitive diagnosis was made. However, the patient remained stable, with no additional medical therapy, for approximately a year. Then, her condition worsened with increasing muscle weakness and a rising CK to 7015 U/L (Fig. 1). Muscle strength examination at this time revealed decreased muscle bulk in the biceps and triceps. She was found to have significantly decreased strength in the hip flexors bilaterally, not capable of lifting her legs against minimal resistance from the examiner. She was unable to rise from a seated position.
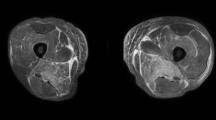
Given the persistent weakness and CK elevation long after statin discontinuation, there was concern for possible statin-associated necrotizing myopathy with consideration for other inflammatory myopathies, such as dermatomyositis (DM) and polymyositis (PM). Review of medication history antecedent to symptom onset revealed no other medications known to cause myopathy (Table 2). Thyroid function was normal, with a thyroid stimulating hormone (TSH) of 3.06 (reference 0.4–5.0 μIU/mL). Given the connection between necrotizing myopathy and malignancy, cancer screening was reviewed. Screening colonoscopy was performed in 2011 with a recommended follow-up of 10 years. Mammogram performed in 2011 was stable from prior with benign findings (BI-RADS 2), and cervical cancer screening in the same year was negative for intraepithelial neoplasm. Repeat muscle biopsy revealed findings similar to the initial biopsy with severe myopathic changes and considerable necrosis (Fig. 2a). Additional immunofluorescence staining using antibody to C5b9 complement membrane attack complex (MAC) and major histocompatibility complex class I (MHC-I) demonstrated the presence of an antibody-mediated attack of non-necrotic fibers, which made statin-induced myopathy and dermatomyositis more likely (Fig. 2b–d). The patient was tested for the presence of novel anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (anti-HMGCR) antibodies with a positive result. She was diagnosed with statin-associated necrotizing autoimmune myopathy (NAM) and started on 40 mg of prednisone daily.
Pathological findings in skeletal muscle (bar = 50 μm). a H&E stain showing a myopathic process with muscle fiber size variability with regenerative and degenerative fibers, the latter indicative of attempts at healing. Prominent necrotic fibers (arrows) and non-necrotic fibers undergoing immune-mediated attack (arrowheads) are noted. The paucity of inflammation (T cells, B cells, Dendritic cells and macrophages) is consistent with statin-induced myopathy compared to PM or DM. b C5b9 (complement membrane attack complex; MAC) immunofluorescence staining showing diffuse (involving sarcolemma and myofiber) upregulation within the necrotic fibers (arrows) and only sarcolemmal upregulation in non-necrotic fibers (arrowheads), a finding seen in antibody-mediated myopathies. c Major histocompatibility complex class I (MHC-I) staining showing upregulation within necrotic (arrow) and non-necrotic (arrowheads) fibers, also indicative of an immune-mediated process. d Spectrin (a protein found on the sarcolemma) immunofluorescence highlights the abnormal pattern of staining in necrotic fibers (arrows) while showing normal pattern of staining in non-necrotic fibers (arrowheads). This confirms that during the early antibody-mediated phase of the disease, the membrane is relatively intact, but in later phases, the membrane integrity is compromised.
Days after initiating corticosteroids, she presented to the hospital with acutely worsened weakness and shortness of breath. In addition to continuing prednisone, intravenous immunoglobulin (IVIG) 2 mg/kg divided into five doses was initiated with improvement in strength. She was also started on methotrexate later in the hospital stay. Upon discharge, she continued treatment with IVIG every four weeks and was maintained on prednisone and methotrexate therapy with improved strength and normalization of CK.
DISCUSSION
Statins are among the most commonly prescribed medications, with 29.7 million people receiving prescriptions for statins in 2005.1 Due to the institution of the new American College of Cardiology and American Heart Association guidelines, the number of patients qualifying for statin therapy has increased substantially, with therapy recommended in 33 million of 101 million people without clinical cardiovascular disease.2 The impact of statins has primarily been in the arena of vascular health, with effects on both primary and secondary prevention of cardiovascular events.3,4 Though these medications are generally well tolerated, they can lead to musculoskeletal side effects, with up to 20 % of patients experiencing myalgias.5 Notably, the estimated incidence of true statin-induced myopathy, characterized by muscle weakness with concomitant elevation in CK, is much lower, at 44 to 120 per million per year.6,7 Anti-HMGCR NAM is even more rare, with an incidence estimated to be 2 per million per year.5 Marked elevation in CK is characteristic of NAM, with a mean value of 10,000 IU/L compared to the self-limited form.5 The mechanism of myocyte disruption in self-limited statin myopathy is not fully understood, but theories include changes in the cholesterol composition of cellular membranes, effects on compounds such as coenzyme Q10 that require cholesterol precursors for synthesis, and decreased isoprenoids responsible for reducing apoptosis of myocytes.7 In general, statins produce a self-limited myopathy that resolves within several months of medication cessation; however, they are also associated with increased incidence of inflammatory myopathies including DM and PM as well as statin-associated NAM.8,9
Similar to self-limited statin myopathy, statin-associated NAM pathophysiology is poorly understood. It is hypothesized that the upregulation of HMGCR in patients taking statins in combination with major histocompatibility complex I (MHC-I) presentation of specific HMGCR antigens leads to the formation of ant-HMGCR antibodies. Furthermore, there is increased expression of HMGCR in regenerating muscle fibers, which may lead to propagation of antibody formation even after statin discontinuation.5 Immunohistochemistry staining of biopsy specimens displays MHC-I upregulation on both necrotic and non-necrotic fibers.10 While normal muscle shows MHC-I antigens only on blood vessels, sarcolemmal and internal upregulation of MHC-I has also been described in other inflammatory myopathies, dystrophinopathies, and other muscular dystrophies.11 Deposition of complement membrane attack complexes on non-necrotic muscle fibers is consistent with the concept that the myofiber degeneration in anti-HMGCR NAM is a complement-mediated antibody-dependent toxicity.12 Additionally, there is an identified association between anti-HMGCR NAM and HLA-DRB1*11.13
The novel anti-HMGCR antibody, which was discovered in 2010, is a promising diagnostic marker for statin-associated NAM.12 Nearly all patients with statin-induced necrotizing autoimmune myopathy have positive anti-HMGCR antibodies with tropism to the catalytic site of HMGCoA reductase. The antibody is not present in the majority of patients with self-limited statin myopathy, or in healthy controls. The reported sensitivity and specificity are 94.4 % and 99.3 %, respectively.14
Not all anti-HMGCR positive NAM patients have been exposed to statins. Approximately 33 % of anti-HMGCR–positive NAM patients have no history of statin use.15 These patients often have slightly different clinical characteristics, such as younger age and increased inflammation, compared to statin-exposed individuals.15
There is no clear association between anti-HMGCR antibody NAM and collagen vascular disease; however, at least two cases have been described: one with anti-synthetase syndrome and another who was Scl-70 positive and was diagnosed with scleroderma.15 Interestingly, our patient tested positive for the anti-centromere antibody, but she did not have other sequelae consistent with a diagnosis of limited scleroderma.
There are several types of NAM, including those associated with malignancy, anti-signal recognition particle (SRP) antibodies, connective tissue disease, HIV and statins.16 Malignancy was less likely in our patient, considering the duration of symptoms for two years, absence of weight loss and normal routine screening. Regardless of the underlying etiology, patients generally present with significant proximal muscle weakness and marked elevation in CK levels, often greater than ten times the upper limit of normal. Electromyography (EMG) shows signs of irritable proximal myopathy, indicative of a severe muscle disease such as an immune-mediated myopathy, compared to a non-irritable myopathy such as a steroid-induced myopathy. Muscle biopsy has features of prominent muscle necrosis with myofiber regeneration and minimal inflammation.8 Myalgias are present in 16–75 % of patients with statin-induced NAM, and peak elevation in CK levels range from 958 to 45,000 IU/L.5 Currently, distinguishing statin-associated NAM from other forms of NAM requires history of statin exposure, consistent muscle biopsy findings, and consideration for and exclusion of alternative causes of NAM. Anti-HMGCR antibody testing is now commercially available from Rheumatology Diagnostics Laboratory, Inc. (RDL) and may be utilized to confirm a diagnosis of statin-associated anti-HMGCR antibody NAM.
There is little clinical evidence for the association between incidence of statin-associated NAM and particular statins or statin dose. Different statins have varied propensities for causing self-limited myopathy, with atorvastatin and simvastatin associated with higher rates of myopathy than rosuvastatin; however, there is no established association between specific statins and the occurrence of statin-associated necrotizing autoimmune myopathy.17,18 Interestingly, our patient was on simvastatin with a normal CK for 10 years and only developed symptoms when transitioned to atorvastatin. In one series of 25 patients, 21 were taking atorvastatin at the time of the development of proximal muscle weakness, though this was likely a representation of prescribing practices and not necessarily indicative of an association between atorvastatin and increased risk of NAM.18 Simvastatin and pravastatin use has also been noted in patients with statin-associated NAM.18 Though there is a known association between statin dose, incidence and severity of self-limited myopathy, a dose relationship with statins in necrotizing autoimmune myopathy has not been established.5,19 Length of statin exposure prior to the development of symptoms has been noted to be approximately 3 years on average, with a range of 9 months to 10 years.18
Immunosuppressive therapy is the mainstay of treatment for statin-associated necrotizing autoimmune myopathy, and is essentially identical to the treatment of other causes of NAM. Currently, there are no controlled trials to guide treatment selection. Initial treatment is generally high-dose prednisone (1 mg/kg/day). Methotrexate is the most commonly used steroid-sparing agent, with azathioprine, mycophenolate mofetil and cyclosporine also being utilized.10 In cases that are refractory to high-dose prednisone and steroid-sparing agents, or in cases that are particularly severe at diagnosis, intravenous immunoglobulin (IVIG) is utilized at a dose of 2 g/kg in divided doses over 2 to 5 days, every 4 to 6 weeks.10 Initial response to therapy is generally quite good with 100 % of patients in two separate studies showing a partial (36 % and 54 %) to complete (64 % and 46 %) response. However, there was also a 50–60 % relapse rate in these studies after tapering immunosuppressive therapy.5,18
Self-limited statin myopathy has been described for several decades, but statin-associated autoimmune necrotizing myopathy is an entity that has been described only in recent years. Though it is rare, statin-associated NAM is an important consideration for the general internist in any patient who has prolonged symptoms of muscle weakness and elevated CK levels after discontinuation of statin therapy. Workup in these cases should include EMG and muscle biopsy, with signs of irritable myopathy on EMG and prominent necrosis with minimal inflammation indicative of necrotizing autoimmune myopathy. Anti-HMGCR antibody testing is now available to aid in confirmation of the diagnosis. Additional studies that examine the effect of different statins and the dose response in statin-associated NAM are needed. Given our patient’s 10-year history of simvastatin use and onset of symptoms only after initiation of atorvastatin therapy, there is reason to suspect relation to therapy with specific statins. Additionally, the severity of her disease at diagnosis and need for aggressive immunosuppressive therapy raises questions regarding the impact of diagnostic delay on treatment effectiveness. Current strategies for immunosuppressive therapy utilizing high-dose prednisone, steroid-sparing agents and IVIG have demonstrated an improvement in strength and CK levels, but clinical research is needed to establish treatment outcomes, determine the role of trending anti-HMGCR antibody titers as a measure of treatment efficacy, and identify the most effective therapeutic regimens.
REFERENCES
Stagnitti M. Trends in Statins Utilization and Expenditures for the U.S. Civilian Noninstitutionalized Population, 2000 and 2005: Statistical Brief #205. Agency for Healthcare Research and Quality Medical Expenditure Panel Survey. 2008.
Loannidis JPA. More than a billion people taking statins? Potential implications of the new cardiovascular guidelines. JAMA. 2014;311(5):463–4.
Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels. JAMA. 1998;279(20):1615–22.
Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled tria. Lancet. 2003;361(9364):1149.
Mohassel P, Mammen AL. Statin-associated autoimmune myopathy and anti-HMGCR autoantibodies: a review. Muscle Nerve. 2013;48(4):477–83.
Graham DJ, Staffa JA, Shatin D, et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90.
Sathasivam S, Lecky B. Statin induced myopathy. BMJ. 2008;337:1159–1162.
Padala S, Thompson PD. Statins as a possible cause of inflammatory and necrotizing myopathies. Atherosclerosis. 2012;222(1):15–21.
Zaraa IR, Labene I, Mrabet D, et al. Simvastatin-induced dermatomyositis in a 50-year-old man. BMJ Case Rep. 2011;29:1–4.
Needham M, Fabian V, Knezevic W. Progressive myopathy with up-regulation of MHC-1 associated with statin therapy. Neuromuscul Disord. 2007;17:194–200.
Dubowitz, V, Sewry, CA, Oldfors, A. Muscle Biopsy: A Practical Approach. 4th Ed. Saunders, Elsevier Ltd; 2013:517.
Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum. 2010;62(9):2757–66.
Mammen AL, Gaudet D, Brisson D, et al. Increased frequency of DRB1*11:01 in anti-hydroxymethylglutaryl-coenzyme A reductase-associated autoimmune myopathy. Arthritis Care Res. 2012;64(8):1233–7.
Mammen AL, Pak K, Williams E, et al. Rarity of anti–3-hydroxy-3-methylglutaryl-coenzyme a reductase antibodies in statin users, including those with self-limited musculoskeletal side effects. Arthritis Care Res. 2012;64(2):269–72.
Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme a reductase (HMGCR) in patients with statin-associated autoimmune myopathy. Arthritis Rheum. 2011;63(3):713–21.
Lianga C, Needhama M. Necrotizing autoimmune myopathy. Curr Opin Rheumatol. 2011;23:612–9.
Molokhia M, McKeigue P, Curcin V, Majeed A. Statin induced myopathy and myalgia: time trend analysis and comparison of risk associated with statin class from 1991–2006. PLoS One. 2008;3(6):e2522.
Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve. 2010;41(2):185–90.
Thompson PD, Clarkson PM, Rosenson RS. An assessment of statin safety by muscle experts. Am J Cardiol. 2006;97(8A):69C–76C.
Acknowledgements
Contributors
We would like to thank Dr. Michael Lawlor for his guidance with the immunofluorescence stains, Cassandra Anderson and Ann Esselman for technical assistance, and Dr. Mike Collins for use of lab resources.
Prior presentations
Society of General Internal Medicine National Meeting, San Diego, CA, 25 April 2014
Conflicts of interest
Dr. Andrew Mammen has patented and licensed the anti-HMGCR antibody test and may receive royalties from INOVA Diagnostics. All other authors declare they do not have a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nichols, L., Pfeifer, K., Mammen, A.L. et al. An Unusual Case of Statin-Induced Myopathy: Anti-HMGCoA Necrotizing Autoimmune Myopathy. J GEN INTERN MED 30, 1879–1883 (2015). https://doi.org/10.1007/s11606-015-3303-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-015-3303-9