Abstract
Background
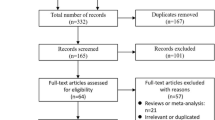
Routine rectal administration of 100 mg of diclofenac or indomethacin was demonstrated to be an effective prevention method to prevent post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis. The systematic review and meta-analysis aimed to estimate the incidence and severity of post-ERCP pancreatitis (PEP) and explore the discrepancies of PEP incidences among different subgroups.
Methods
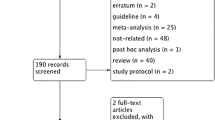
The PubMed, Web of Science, and Ovid EMBASE databases were searched for studies published until December 2020. Only randomized controlled trials (RCTs) reported rectal administration of 100 mg or higher doses of diclofenac or indomethacin, with PEP as the primary outcomes were eligible for inclusion. The overall and severity of PEP were estimated. Subgroup analysis was performed based on geographic regions, risk level, study beginning time, type of NSAIDs, administration time, and sample size.
Results
There were 26 randomized controlled trials (RCTs) with 7954 patients in 31 NSAIDs arms. The pooled incidences were 7.2% for overall PEP (95% confidence interval (CI) 5.9–8.5%), 5.0% for mild PEP (95% CI, 4.0–6.0%), and 1.5% for moderate and severe PEP (0.8–2.3%). PEP rate were higher in patients receiving rectal indomethacin than that of patients receiving rectal diclofenac (7.8% (95% CI, 6.4–9.3%) vs 3.8% (95% CI, 2.2–5.3%), p = 0.009). The PEP rates of high-risk patients and average-risk patients were 8.9% (95% CI, 5.6–12.2%) and 6.4% (95% CI, 5.1–7.6%), respectively (p = 0.160).
Conclusions
The incidence of PEP was higher in patients receiving 100 mg rectal indomethacin than patients receiving 100 mg diclofenac. The effect of 100 mg diclofenac versus indomethacin on preventing PEP requires further study.


Similar content being viewed by others
Abbreviations
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- PEP:
-
Post-ERCP pancreatitis
- NSAID:
-
Nonsteroidal anti-inflammatory drugs
- ESGE:
-
European Society of Gastrointestinal Endoscopy
- RCT:
-
Randomized controlled trial
- CI:
-
Confidence interval
- PD:
-
Pancreatic duct
- RR:
-
Risk ratio
References
Andriulli A, Loperfido S, Napolitano G, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol 2007;102:1781-8.
Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc 2015;81:143-149 e9.
Mutneja HR, Vohra I, Go A, et al. Temporal trends and mortality of post-ERCP pancreatitis in the United States: a nationwide analysis. Endoscopy 2021;53:357-366.
Elmunzer BJ, Scheiman JM, Lehman GA, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med 2012;366:1414-22.
Luo H, Zhao L, Leung J, et al. Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography: a multicentre, single-blinded, randomised controlled trial. Lancet 2016;387:2293-2301.
Choudhary A, Bechtold ML, Arif M, et al. Pancreatic stents for prophylaxis against post-ERCP pancreatitis: a meta-analysis and systematic review. Gastrointest Endosc 2011;73:275-82.
Park CH, Paik WH, Park ET, et al. Aggressive intravenous hydration with lactated Ringer's solution for prevention of post-ERCP pancreatitis: a prospective randomized multicenter clinical trial. Endoscopy 2018;50:378-385.
Bang UC, Nojgaard C, Andersen PK, et al. Meta-analysis: Nitroglycerin for prevention of post-ERCP pancreatitis. Aliment Pharmacol Ther 2009;29:1078-85.
Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - updated June 2014. Endoscopy 2014;46:799-815.
Geraci G, Palumbo VD, D'Orazio B, et al. Rectal Diclofenac administration for prevention of post-Endoscopic Retrograde Cholangio-Pancreatography (ERCP) acute pancreatitis. Randomized prospective study. Clin Ter 2019;170:e332-e336.
Leerhoy B, Nordholm-Carstensen A, Novovic S, et al. Diclofenac is associated with a reduced incidence of post-endoscopic retrograde cholangiopancreatography pancreatitis: results from a Danish cohort study. Pancreas 2014;43:1286-90.
Fogel EL, Lehman GA, Tarnasky P, et al. Rectal indometacin dose escalation for prevention of pancreatitis after endoscopic retrograde cholangiopancreatography in high-risk patients: a double-blind, randomised controlled trial. Lancet Gastroenterol Hepatol 2020;5:132-141.
Kang X, Zheng L, Zeng W, et al. Risk Factors for Post-ERCP Pancreatitis in High-Risk Patients Receiving Post-procedure Rectal Indomethacin. J Gastrointest Surg 2018;22:1903-1910.
Leerhoy B, Nordholm-Carstensen A, Novovic S, et al. Effect of body weight on fixed dose of diclofenac for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Scand J Gastroenterol 2016;51:1007-12.
Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc 1991;37:383-93.
Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12.
Oremus M, Wolfson C, Perrault A, et al. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord 2001;12:232-6.
Hou YC, Hu Q, Huang J, et al. Efficacy and safety of rectal nonsteroidal anti-inflammatory drugs for prophylaxis against post-ERCP pancreatitis: a systematic review and meta-analysis. Sci Rep 2017;7:46650.
Thiruvengadam NR, Forde KA, Ma GK, et al. Rectal Indomethacin Reduces Pancreatitis in High- and Low-Risk Patients Undergoing Endoscopic Retrograde Cholangiopancreatography. Gastroenterology 2016;151:288-297 e4.
Serrano JPR, de Moura DTH, Bernardo WM, et al. Nonsteroidal anti-inflammatory drugs versus placebo for post-endoscopic retrograde cholangiopancreatography pancreatitis: a systematic review and meta-analysis. Endosc Int Open 2019;7:E477-E486.
Inamdar S, Han D, Passi M, et al. Rectal indomethacin is protective against post-ERCP pancreatitis in high-risk patients but not average-risk patients: a systematic review and meta-analysis. Gastrointest Endosc 2017;85:67-75.
Ahmad D, Lopez KT, Esmadi MA, et al. The effect of indomethacin in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a meta-analysis. Pancreas 2014;43:338-42.
Elmunzer BJ, Waljee AK, Elta GH, et al. A meta-analysis of rectal NSAIDs in the prevention of post-ERCP pancreatitis. Gut 2008;57:1262-7.
Mohammad Alizadeh AH, Abbasinazari M, Hatami B, et al. Comparison of rectal indomethacin, diclofenac, and naproxen for the prevention of post endoscopic retrograde cholangiopancreatography pancreatitis. Eur J Gastroenterol Hepatol 2017;29:349-354.
Makela A, Kuusi T, Schroder T. Inhibition of serum phospholipase-A2 in acute pancreatitis by pharmacological agents in vitro. Scand J Clin Lab Invest 1997;57:401-7.
Takada Y, Bhardwaj A, Potdar P, et al. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene 2004;23:9247-58.
Avila P, Holmes I, Kouanda A, et al. Practice patterns of post-ERCP pancreatitis prophylaxis techniques in the United States: a survey of advanced endoscopists. Gastrointest Endosc 2020;91:568-573 e2.
Funding
This work was supported in part by the National Natural Science Foundation of China (81970557 and 82003152). The funding bodies were not involved in the design of this study, in the collection, analysis, and interpretation of the data, or in writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: Xiaoyu Kang, Yanglin Pan, Xuegang Guo. Data curation: Xiaoyang Guo, Zhangqian Chen, Hui Luo. Formal analysis: Lijun Lou. Methodology: Jieya Lu. Software: Zhirui Zhou. Writing—original draft: Xiaoyu Kang. Writing—review and editing: Yanglin Pan.
Corresponding author
Ethics declarations
Ethics Approval
An ethical approval was not required for this meta-analysis after we consulted with our institutional ethical board of our hospital. So an ethical board approval statement was not included in this article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.










Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, X., Guo, X., Chen, Z. et al. The Incidence and Severity of Post-ERCP Pancreatitis in Patients Receiving Standard Administration of NSAIDs: a Systematic Review and Meta-analysis. J Gastrointest Surg 26, 2380–2389 (2022). https://doi.org/10.1007/s11605-022-05399-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-022-05399-6