Abstract
Purpose
Careful patient selection plays a crucial role in avoiding overtreatment and further increases survival rates in patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal cancer (CRC) with peritoneal metastases (PM).
Methods
The clinical and molecular factors influencing survival in patients who had undergone CRS with HIPEC between January 2015 and December 2018 were analyzed.
Results
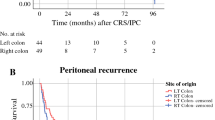
Sixty-six patients underwent CRS with HIPEC during the study period. The median overall survival (OS) was 36 months, with a 3-year OS of 43%. Multivariate analysis revealed increased PCI (HR: 1.21; 95% CI: 1.02–1.41; p = 0.020), right-sided primary tumor (HR: 3.01; 95% CI: 1.27–7.13; p = 0.017), and BRAF V600E mutation (HR: 4.55; 95% CI: 1.21–17.21; p = 0.025) as independent predictors for worse OS.
Conclusion
In addition to confirming the prognostic role of PCI, our study extends the role of BRAF mutation and right primary tumor location as markers for worse prognosis.

Similar content being viewed by others
References
Franko J, Shi Q, Meyers JP, Maughan TS, Adams RA, Seymour MT, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol 2016;17:1709–19. https://doi.org/10.1016/S1470-2045(16)30500-9.
Goéré D, Sourrouille I, Gelli M, Benhaim L, Faron M, Honoré C. Peritoneal Metastases from Colorectal Cancer: Treatment Principles and Perspectives. Surg Oncol Clin N Am 2018;27:563–583. https://doi.org/10.1016/j.soc.2018.02.011.
Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: A pooled analysis of North Central Cancer Treatment Group phase III trials N9741 and N9841. J Clin Oncol 2012;30:263–7. https://doi.org/10.1200/JCO.2011.37.1039.
Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, et al. Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for the Management of Peritoneal Carcinomatosis from Colorectal Cancer: A Multi-Institutional Study. J Clin Oncol 2004;22:3284–92. https://doi.org/10.1200/JCO.2004.10.012.
Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681–5. https://doi.org/10.1200/JCO.2008.19.7160.
Verwaal VJ, van Ruth S, de Bree E, van Slooten GW, van Tinteren H, Boot H, et al. Randomized Trial of Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy Versus Systemic Chemotherapy and Palliative Surgery in Patients With Peritoneal Carcinomatosis of Colorectal Cancer. J Clin Oncol 2003;21:3737–43. https://doi.org/10.1200/JCO.2003.04.187.
Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu R-H, Kim TW, Ismail F, Tan IB, Yeh K-H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol Off J Eur Soc Med Oncol 2018; 29:44–70. https://doi.org/10.1093/annonc/mdx738.
Version 2.2020 (03 March 2020). Colon Cancer. Clinical Practice Guidelines in Oncology (NCCN Guidelines) n.d.
Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:256–66. https://doi.org/10.1016/S1470-2045(20)30599-4.
P H Sugarbaker, A M Averbach, P Jacquet, O A Stuart ADS. Hyperthermic Intraoperative Intreperitoneal Chemotherapy (HIIC) With Mitomycin C. Surg Technol Int 1996;5:245–9.
Sugarbaker PH. Peritonectomy procedures. Perit Carcinomatosis A Multidiscip Approach 2007;221:247–64. https://doi.org/10.1007/978-0-387-48993-3_15.
Rotolo S, Di Giorgio A, Santullo F, Attalla El Halabieh M, Lodoli C, Abatini C, et al. Cytoreductive surgery and mitomycin C hyperthermic intraperitoneal chemotherapy with CO2 recirculation (HIPEC-CO2) for colorectal cancer peritoneal metastases: analysis of short-term outcomes. Updates Surg 2021. https://doi.org/10.1007/s13304-021-01034-2.
Segelman J, Granath F, Holm T, MacHado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 2012;99:699–705. https://doi.org/10.1002/bjs.8679.
Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann Surg 2009;250:187–96. https://doi.org/10.1097/SLA.0b013e3181b13ca2.
Huang CQ, Min Y, Wang SY, Yang XJ, Liu Y, Xiong B, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: A systematic review and meta-analysis of current evidence. Oncotarget 2017;8:55657–83. https://doi.org/10.18632/oncotarget.17497.
Waite K, Youssef H. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann Surg Oncol 2017;24:705–20. https://doi.org/10.1245/s10434-016-5712-3.
Elias D. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric french study. J Clin Oncol 2010;28:63–8. https://doi.org/10.1200/JCO.2009.23.9285.
Verwaal VJ, Van Ruth S, Witkamp A, Boot H, Van Slooten G, Zoetmulder FAN. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005;12:65–71. https://doi.org/10.1007/s10434-004-1167-z.
Faron M, Macovei R, Goéré D, Honoré C, Benhaim L, Elias D. Linear Relationship of Peritoneal Cancer Index and Survival in Patients with Peritoneal Metastases from Colorectal Cancer. Ann Surg Oncol 2016;23:114–9. https://doi.org/10.1245/s10434-015-4627-8.
Sugarbaker PH. Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol Suppl 1999;43:15–25. https://doi.org/10.1007/s002800051093.
Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, et al. Extent of Colorectal Peritoneal Carcinomatosis: Attempt to Define a Threshold Above Which HIPEC Does Not Offer Survival Benefit: A Comparative Study. Ann Surg Oncol 2015;22:2958–64. https://doi.org/10.1245/s10434-015-4387-5.
Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer a systematic review and meta-analysis. JAMA Oncol 2017;3:211–9. https://doi.org/10.1001/jamaoncol.2016.4227.
Nitsche U, Stögbauer F, Späth C, Haller B, Wilhelm D, Friess H, et al. Right sided colon cancer as a distinct histopathological subtype with reduced prognosis. Dig Surg 2016;33:157–63. https://doi.org/10.1159/000443644.
Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer 2015;121:830–5. https://doi.org/10.1002/cncr.29129.
Creasy JM, Sadot E, Koerkamp BG, Chou JF, Gonen M, Kemeny NE, et al. The Impact of Primary Tumor Location on Long-Term Survival in Patients Undergoing Hepatic Resection for Metastatic Colon Cancer. Ann Surg Oncol 2018;25:431–8. https://doi.org/10.1245/s10434-017-6264-x.
Dupré A, Malik HZ, Jones RP, Diaz-Nieto R, Fenwick SW, Poston GJ. Influence of the primary tumour location in patients undergoing surgery for colorectal liver metastases. Eur J Surg Oncol 2018;44:80–6. https://doi.org/10.1016/j.ejso.2017.10.218.
Kelly KJ, Alsayadnasser M, Vaida F, Veerapong J, Baumgartner JM, Patel S, et al. Does Primary Tumor Side Matter in Patients with Metastatic Colon Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy? Ann Surg Oncol 2019;26:1421–7. https://doi.org/10.1245/s10434-019-07255-5.
Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer 2017;84:69–80. https://doi.org/10.1016/j.ejca.2017.07.016.
Andreyev HJN, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: The “RASCAL II” study. Br J Cancer 2001;85:692–6. https://doi.org/10.1054/bjoc.2001.1964.
Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. RAF/ RAS oncogenes and mismatch-repair status 2002;418:2002.
Roth AD, Tejpar S, Delorenzi M, Yan P, Fiocca R, Klingbiel D, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: Results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol 2010;28:466–74. https://doi.org/10.1200/JCO.2009.23.3452.
Blons H, Emile JF, Le Malicot K, Julié C, Zaanan A, Tabernero J, et al. Prognostic value of KRAS mutations in stage III colon cancer: Post hoc analysis of the PETACC8 phase III trial dataset. Ann Oncol 2014;25:2378–85. https://doi.org/10.1093/annonc/mdu464.
Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VEPP, Rutten HJT, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol 2010;21:2396–402. https://doi.org/10.1093/annonc/mdq258.
Orlandi A, Calegari MA, Inno A, Berenato R, Caporale M, Niger M, et al. BRAF in metastatic colorectal cancer: the future starts now. Pharmacogenomics 2015;16:2069–81. https://doi.org/10.2217/pgs.15.140.
Sanz-Garcia E, Argiles G, Elez E, Tabernero J. BRAF mutant colorectal cancer: Prognosis, treatment, and new perspectives. Ann Oncol 2017;28:2648–57. https://doi.org/10.1093/annonc/mdx401.
Schneider MA, Eden J, Pache B, Laminger F, Lopez-Lopez V, Steffen T, et al. Mutations of RAS/RAF proto-oncogenes impair survival after cytoreductive surgery and HIPEC for peritoneal metastasis of colorectal origin. Ann Surg 2018;268:845–53. https://doi.org/10.1097/SLA.0000000000002899.
Graf W, Cashin PH, Ghanipour L, Enblad M, Botling J, Terman A, et al. Prognostic Impact of BRAF and KRAS Mutation in Patients with Colorectal and Appendiceal Peritoneal Metastases Scheduled for CRS and HIPEC. Ann Surg Oncol 2020;27:293–300. https://doi.org/10.1245/s10434-019-07452-2.
Guinney J, Dienstmann R, Wang X, De Reyniès A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–6. https://doi.org/10.1038/nm.3967.
Ubink I, van Eden WJ, Snaebjornsson P, Kok NFM, van Kuik J, van Grevenstein WMU, Laclé MM, Sanders J, Fijneman RJA, Elias SG, et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases.Br J Surg 2018;105:e204–e211. https://doi.org/10.1002/bjs.10788.
Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: Integrating the consensus molecular subtypes. JNCCN J Natl Compr Cancer Netw 2017;15:411–9. https://doi.org/10.6004/jnccn.2017.0038.
Cannon E, Buechler S. Colon Cancer Tumor Location Defined by Gene Expression May Disagree With Anatomic Tumor Location. Clin Colorectal Cancer 2019;18:149–58. https://doi.org/10.1016/j.clcc.2019.02.002.
Fujiyoshi K, Yamamoto G, Takahashi A, Arai Y, Yamada M, Kakuta M, et al. High concordance rate of KRAS/BRAF mutations and MSI-H between primary colorectal cancer and corresponding metastases. Oncol Rep 2017;37:785–92. https://doi.org/10.3892/or.2016.5323.
Author information
Authors and Affiliations
Contributions
FS, SR, AD, and FP contributed to study conception and design. CA, MAEH, and MAC contributed to acquisition of data. FS, MAEH, and SR contributed to writing the manuscript. MAEH, CL, and MM contributed to drafting of the manuscript. FS, AD, and FP contributed to critical revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the local Institutional Review Board (IRB).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Di Giorgio, A., Santullo, F., Attalla El Halabieh, M. et al. Clinical and Molecular Features in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinosis from Colorectal Cancer. J Gastrointest Surg 25, 2649–2659 (2021). https://doi.org/10.1007/s11605-021-05073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-021-05073-3