Abstract
Background
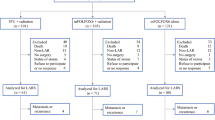
Neoadjuvant chemoradiation (CRT) impairs bowel function in patients with rectal cancer treated with total mesorectal excision (TME). The impact of other forms of neoadjuvant therapy such as neoadjuvant chemotherapy alone (NC) and induction chemotherapy followed by CRT (total neoadjuvant therapy or TNT) on postoperative bowel function has not been investigated.
Methods
We conducted a retrospective review of 176 rectal cancer patients treated between November 1, 2011, and August 31, 2017. All patients completed the MSKCC Bowel Function Instrument (BFI), a validated bowel function questionnaire, at least 6 months after TME and/or ileostomy reversal. Differences in BFI scores were compared across four groups (surgery alone, CRT, NC, and TNT) and also according to exposure to neoadjuvant RT and neoadjuvant chemotherapy. A multivariable linear regression model was used to evaluate the independent relationship between exposure to neoadjuvant RT or chemotherapy and BFI.
Results
BFI total scores were significantly different between the four groups (p = 0.008). Exposure to RT correlated with worse BFI total scores (p = 0.002), and no differences were found in BFI total score after exposure to neoadjuvant chemotherapy (p = 0.92). In a linear regression model, only exposure to RT (β = − 5.1; 95% CI − 8.9 to − 1.3; p = 0.008) and tumor distance from the anal verge (β = 1.23; 95% CI 0.48 to 1.97; p = 0.001) were significantly correlated with BFI total score.
Conclusion
NC, whether administered alone or added to CRT, does not seem to impair bowel function. These data should be used to counsel rectal cancer patients when discussing neoadjuvant therapy options.


Similar content being viewed by others
Change history
24 April 2023
This article has been updated. All author names have been corrected.
References
Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998;133:894–899.
Kapiteijn E, Putter H, van de Velde CJH, Cooperative investigators of the Dutch ColoRectal Cancer Group. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 1998;89:1142–1149. https://doi.org/10.1046/j.1365-2168.2002.02196.x
van Gijn W, Marijnen CAM, Nagtegaal ID, Kranenbarg EM-K, Putter H, Wiggers T, Rutten HJT, Påhlman L, Glimelius B, van de Velde CJH, Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–582. https://doi.org/10.1016/S1470-2045(11)70097-3
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab H-R, Villanueva M-T, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926–1933. https://doi.org/10.1200/JCO.2011.40.1836
Engel AF, Oomen JLT, Eijsbouts Q A. J, Cuesta MA, van de Velde CJH. Nationwide decline in annual numbers of abdomino-perineal resections: effect of a successful national trial? Colorectal Dis 2003;5:180–184. https://doi.org/10.1046/j.1463-1318.2003.00454.x
Tilney HS, Heriot AG, Purkayastha S, Antoniou A, Aylin P, Darzi AW, Tekkis PP. A national perspective on the decline of abdominoperineal resection for rectal cancer. Ann Surg 2008;247:77–84. https://doi.org/10.1097/SLA.0b013e31816076c3
Loos M, Quentmeier P, Schuster T, Nitsche U, Gertler R, Keerl A, Kocher T, Friess H, Rosenberg R. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:1816–1828. https://doi.org/10.1245/s10434-012-2827-z
Scheer AS, Boushey RP, Liang S, Doucette S, O’Connor AM, Moher D. The long-term gastrointestinal functional outcomes following curative anterior resection in adults with rectal cancer: a systematic review and meta-analysis. Dis Colon Rectum 2011;54(12):1589–1597. https://doi.org/10.1097/DCR.0b013e3182214f11
Bryant CLC, Lunniss PJ, Knowles CH, Thaha MA, Chan CLH. Anterior resection syndrome. Lancet Oncol. 2012;13(9):e403–408. https://doi.org/10.1016/S1470-2045(12)70236-X
Emmertsen KJ, Laurberg S, Rectal Cancer Function Study Group. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg 2013;100:1377–1387. https://doi.org/10.1002/bjs.9223
Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis 2013;15:1130–9. https://doi.org/10.1111/codi.12244
Peeters KCMJ, van de Velde CJH, Leer JWH, Martijn H, Junggeburt JMC, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CAM. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patiens—a Dutch Colorectal Cancer Group study. J Clin Oncol 2005;23:6199–6206. https://doi.org/10.1200/JCO.2005.14.779
Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG, Temple LKF, Paty PB, Saltz LB. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32:513–518. https://doi.org/10.1200/JCO.2013.51.7904
Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol 2018, in press. https://doi.org/10.1001/jamaoncol.2018.0071
Haller DG, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Gilberg F, Rittweger K, Schmoll H-J. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–1471. https://doi.org/10.1200/JCO.2010.33.6297
Schmoll H-J, Twelves C, Sun W, O’Connell MJ, Cartwright T, McKenna E, Saif M, Lee S, Yothers G, Haller D. Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controlled trials. Lancet Oncol 2014;15:1481–1492. https://doi.org/10.1016/S1470-2045(14)70486-3
Schmoll H-J, Tabernero J, Maroun J, de Braud F, Price T, Van Cutsem E, Hill M, Hoersch S, Rittweger K, Haller DG. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–3740. https://doi.org/10.1200/JCO.2015.60.9107
Yothers G, O’Connell MJ, Allegra CJ, Kuebler JP, Colangelo LH, Petrelli NJ, Wolmark N. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–3774. https://doi.org/10.1200/JCO.2011.36.4539
Temple LK, Bacik J, Savatta SG, Gottesman L, Paty PB, Weiser MR, Guillem JG, Minsky BD, Kalman M, Thaler HT, Schrag D, Wong DW. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum 2005;48:1353–1365. https://doi.org/10.1007/s10350-004-0942-z
Chen TY-T, Emmertsen KJ, Laurberg S. What are the best questionnaires to capture anorectal function after surgery in rectal cancer? Curr Colorectal Cancer Rep 2015;11:37–43. https://doi.org/10.1007/s11888-014-0217-6
Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. https://doi.org/10.1016/S0140-6736(07)61602-X
Kanat O, Ertas H, Caner B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J Clin Oncol 2017;8:329–335. https://doi.org/10.5306/wjco.v8.i4.329
Arias F, Viudez A, Eito C, Ibanez-Beroiz B, Asin G, Hern I, ez, Cambra K, Errasti M, Barrado M, Campo M, Visus I, Flamarique S, Ciga MA. Effects of adjuvant oxaliplatin on anal function in locally advanced rectal cancer treated with preoperative chemo-radiotherapy and low anterior resection. Colorectal Cancer 2017;3:1. https://doi.org/10.21767/2471-9943.100033
Qin Q, Huang B, Cao W, Zhou J, Ma T, Zhou Z, Wang J, Wang L. Bowel dysfunction after low anterior resection with neoadjuvant chemoradiotherapy or chemotherapy alone for rectal cancer: a cross-sectional study from China. Dis Colon Rectum 2017;60:697–705. https://doi.org/10.1097/DCR.0000000000000801
Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922–928. https://doi.org/10.1097/SLA.0b013e31824f1c21
PROSPECT: chemotherapy alone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery. https://clinicaltrials.gov/ct2/show/NCT01515787. Accessed 12 May 2018
Emmertsen KJ, Laurberg S. Bowel dysfunction after treatment for rectal cancer. Acta Oncol 2008;47:994–1003. https://doi.org/10.1080/02841860802195251
Hupkens BJP, Martens MH, Stoot JH, Berbee M, Melenhorst J, Beets-Tan RG, Beets GL, Breukink SO. Quality of life in rectal cancer patients after chemoradiation: watch-and-wait policy versus standard rsection - a matched-controlled study. Dis Colon Rectum 2017;60:1032–1040. https://doi.org/10.1097/DCR.0000000000000862
Juul T, Elfeki H, Christensen P, Laurberg S, Emmertsen KJ, Bager P. Normative data for the low anterior resection syndrome score (LARS score). Ann Surg 2018, in press. https://doi.org/10.1097/SLA.0000000000002750
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, Guillem JG, Paty PB, Avila K, Garcia-Aguilar J, Rectal Cancer Consortium. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer 2015;15:767. https://doi.org/10.1186/s12885-015-1632-z
Funding
NCI grant P30 CA008748
Author information
Authors and Affiliations
Contributions
Dr. Quezada-Diaz was responsible for the design, data collection, statistical analysis, manuscript writing, and final approval of this version and agrees to be accountable for all aspects of the work. Dr. Jimenez-Rodriguez was responsible for study design, data collection, manuscript writing, and final approval of this version and agrees to be accountable for all aspects of the work. Drs. Pappou and Smith were responsible for study design, manuscript writing, and final approval of this version and agree to be accountable for all aspects of the work. Dr. Patil was responsible for statistical analysis, manuscript writing, and final approval of this version and agrees to be accountable for all aspects of the work. Drs. Wei, Guillem, Paty, Nash, and Weiser were responsible for interpretation of data, manuscript writing, and final approval of this version and agree to be accountable for all aspects of the work. Dr. Garcia-Aguilar was responsible for study design, manuscript writing, and final approval of this version and agrees to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
The study was approved by the institutional review board of Memorial Sloan Kettering Cancer Center, and a waiver of informed consent was obtained.
Appendix. MSK Bowel Function Instrument
Appendix. MSK Bowel Function Instrument


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quezada-Diaz, F., Jimenez-Rodriguez, R.M., Pappou, E.P. et al. Effect of Neoadjuvant Systemic Chemotherapy With or Without Chemoradiation on Bowel Function in Rectal Cancer Patients Treated With Total Mesorectal Excision. J Gastrointest Surg 23, 800–807 (2019). https://doi.org/10.1007/s11605-018-4003-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-018-4003-7