Abstract
Objective
This study aims to evaluate the development of an artificial neural network (ANN) method for predicting the survival likelihood of pancreatic adenocarcinoma patients. The ANN predictive model should produce results with a 90% sensitivity.
Methods
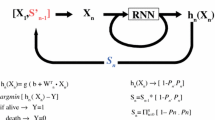
A prospective examination of the records for 283 consecutive pancreatic adenocarcinoma patients is used to identify 219 records with complete data. These records are then used to create two unique samples which are then used to train and validate an ANN predictive model. Numerous network architectures are evaluated, following recommended ANN development protocols.
Results
Several backpropagation-trained ANNs were produced that satisfied the 90% sensitivity requirement. An ANN model with over a 91% sensitivity is selected because even though it did not have the highest sensitivity, it was able to achieve over 38% specificity.
Conclusions
ANN models can accurately predict the 7-month survival of pancreatic adenocarcinoma patients, both with and without resection, at a 91% sensitivity and 38% specificity. This implies that ANN models may be useful objective decision tools in complex treatment decisions. This information may be used by patients and surgeons in determining optimal treatment plans that minimize regret and improve the quality of life for these patients.


Similar content being viewed by others
References
Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: challenge of the facts. World J Surg 2003;27:1075–84.
Fesinmeyer MD, Austin MA, Li CI, De Roos AJ, Bowen DJ. Differences in survival by histologic type of pancreatic cancer. Cancer Epidemiol Biomark Prev 2005;14:1766–73.
Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control 2006;17:403–9.
Kuhlmann KF, de Castro SM, Wesseling JG, ten Kate FJ, Offerhaus GJ, Busch OR, Van Gulik TM, Obertop H, Gouma DJ. Surgical treatment of pancreatic adenocarcinoma: actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–58.
Bradley EL III. Long-term survival after pancreaticoduodenectomy for ductal adenocarcinoma: the emperor has no clothes? Pancreas 2008;37:349–351
McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, Anderson FA, Tseng JF. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg 2007;246:246–53.
Vollmer CM, Sanchez N, Gondek S, McAuliffe J, Kent TS, Christein JD, Callery MP, Pancreatic Surgery Mortality Study Group. A root-cause analysis of mortality following major pancreatectomy. J Gastrointest Surg 2012;16:89–103.
Miura T, Hirano S, Nakamura T, et al. A new preoperative prognostic scoring system to predict prognosis in patients with locally advanced pancreatic body cancer who undergo distal pancreatectomy with en bloc celiac axis resection: a retrospective cohort study. Surgery 2014;155:457–467
Abramson MA, Swanson EW, Whang EE. Surgical resection versus palliative chemoradiotherapy for the management of pancreatic cancer with local venous invasion: a decision analysis. J Gastrointest Surg 2009;13:26–34
Joliat GR, Peterman D, Demartines N, Schafer M. External assessment of the Early Mortality Risk Score in patient with adenocarcinoma undergoing pancreaticoduodenectomy. HPB 2015;17:605–610
Dasari BV, Roberts KJ, Hodson J, et al. A model to predict survival following pancreaticoduodenectomy for malignancy based on tumour site, stage and lymph node ratio. HPB 2016;18:332–338
de Geus SW, Evans DB, Bliss LA, et al. Neoadjuvant therapy versus upfront surgical strategies in resectable pancreatic cancer: a Markov decision analysis. Eur J Surg Oncol 2016;42:1552–1560
Jamal MH, Doi SA, Moser AJ, et al. McGill Brisbane System Score for patients with resectable pancreatic head adenocarcinoma. World J Gastroenterol 2014 20:12226–12232
Regulin-Coyne E, Carrooll JE, Smith JK, et al. Perioperative mortality after pancreatectomy: a risk score to aid decision-making. Surgery 2012;152(3 Suppl 1):S120-S127
Abott DE, Tzeng CW, Merkow RP, et al. The cost-effectiveness of neoadjuvant chemoradiation is superior to a surgery-first approach in the treatment of pancreatic head adenocarcinoma. Ann Surg Oncol 2013;20 Suppl 3:S500-S508
Sutton JM, Wilson GC, Paquette IM, et al. Cost-effectiveness after pancreaticoduodenectomy: bolstering the volume argument. HPB 2014;16:1056–1061
Morris S, Gurusamy KS, Sherringham J, Davidson BR. Cost-effectiveness of diagnostic laparoscopy for assessing resectability in pancreatic and perampullary cancer. BMC Gastroenterol 2015 15:44
Velanovich V. The association of quality-of-life measures with malignancy and survival in patients with pancreatic pathology. Pancreas 2011;40:1063–9
Mogal H, Vermilion SA, Dodson R, et al. Modified frailty index predicts morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol 2017;24:1714–1721
Hernandez JM, Tsalatsanis A, Humphries LA, et al. Defining optimum treatment of patients with pancreatic adenocarcinoma using regret-based decision curve analysis. Ann Surg 2014;259:1208–14
Shin SA, Kim SC, Hong SM, et al. Can statistically determined prognostic factors predict the long-term survival of patients with pancreatic ductal adenocarcinoma following resection? Clinicopathological analysis of 82 long-term survivors. Pancreas 2014;43:571–577
Lisboa PJ, Taktak AF. The use of artificial neural networks in decision support in cancer: a systematic review. Neural Netw 2006;19:408–15.
Bollschweiler EH, Mönig SP, Hensler K, et al. Artificial neural network for prediction of lymph node in gastric cancer: a phase II diagnostic study. Ann Surg Oncol 2004;11:506–11.
Pofahl WE, Walczak SM, Rhone E, Izenberg SD. Use of an artificial neural network to predict length of stay in acute pancreatitis. Am Surg 1998;64:868–72.
Santos-Garcıa G, Varela G, Novoa N, Jiménez MF. Prediction of postoperative morbidity after lung resection using an artificial neural network ensemble. Art Intell Med 2004;30:61–9.
Syed Z, Rubinfeld I, Patton JH, et al. Using procedural codes to supplement risk adjustment: a nonparametric learning approach. J Am Coll Surg 2011;212:1086–93.
Bartosch-Härlid A, Andersson B, Aho U, et al. Artificial neural networks in pancreatic disease. Br J Surg 2008;95:817–26.
Swanson RS, Pezzi CM, Mallin K, et al. The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the National Cancer Data Base. Ann Surg Oncol 2014;21:4059–67
Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586–94
Walczak S, Cerpa N. Heuristic principles for the design of artificial neural networks. Inf Softw Technol 1999;41:107–17
Zhang GP. Avoiding pitfalls in neural network research. IEEE Trans Syst Man Cybern Part C Appl Rev. 2007;37:3–16
Barnard, E., & Wessels, L. F. A. (1992). Extrapolation and interpolation in neural network classifiers. IEEE Control Syst, 12(5), 50–53.
Specht, D. F. (1991). A general regression neural network. IEEE Trans Neural Netw, 2(6), 568–576.
De Villiers, J., & Barnard, E. (1993). Backpropagation neural nets with one and two hidden layers. IEEE Trans Neural Netw, 4(1), 136–141.
Sexton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg 2011;253:1223–1229
Velanovich V, Wollner I. Quality of life and performance status in patients with pancreatic and periampullary tumors. Int J Clin Oncol 2011;16:401–407
Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol 2008;26:1355–63.
Polistina F, Costantin G, Casamassima F, Francescon P, Guglielmi R, Panizzoni G, Febbraro A, Ambrosino G. Unresectable locally advanced pancreatic cancer: a multimodal treatment using neoadjuvant chemoradiotherapy (gemcitabine plus stereotactic radiosurgery) and subsequent surgical exploration. Ann Surg Oncol 2010;17:2092–101.
Robinson DW Jr, Eisenberg DF, Cella D, Zhao N, de Boer C, DeWitte M. The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. J Support Oncol 2008;6:283–90.
Walczak S. Evaluating medical decision making heuristics and other business heuristics with neural networks. In Intelligent decision making: an AI-based approach, pp. 259–287, Springer Berlin, Heidelberg, 2008.
Statement of Author Contributions
For both Steven Walczak and Vic Velanovich, each have made substantial contributions to the conception or design of the work; the acquisition, analysis, and interpretation of data for the work; AND drafting the work or revising it critically for important intellectual content; AND have given final approval of the version to be published; AND are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
This research is unfunded and the authors report no conflict of interest.
Rights and permissions
About this article
Cite this article
Walczak, S., Velanovich, V. An Evaluation of Artificial Neural Networks in Predicting Pancreatic Cancer Survival. J Gastrointest Surg 21, 1606–1612 (2017). https://doi.org/10.1007/s11605-017-3518-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-017-3518-7