Abstract
Purpose
Until March 2018, patients with high-risk localized prostate cancer had been administered high-dose-rate brachytherapy (HDR-BT) combined with external beam radiotherapy (EBRT) without additional hormone therapy (HT) at our institution. In this study, we aimed to evaluate long-term outcomes of this treatment.
Materials and methods
Patients with prostate cancer who received HDR-BT and EBRT between April 1997 and March 2021 and who were followed up for at least 6 months were included in the study. High-risk groups were classified into five levels according to the National Comprehensive Cancer Network guidelines. The EBRT and HDR-BT doses were 39–45 Gy/13–25 fractions. and 16.5–22 Gy/2–4 fractions, respectively. None of the patients received HT during initial treatment. The Kaplan–Meier method was used to estimate biochemical freedom from failure (bFFF), cause-specific survival (CSS), and overall survival (OS) rates. Biochemical failure was also determined.
Results
Seventy-two patients were enrolled in the study, with a median follow-up of 91.9 months. The median age and initial prostate-specific antigen (iPSA) level were 71 years and 10.95 ng/mL, respectively. The median biologically effective dose for HDR-BT plus EBRT was 270.3 Gy. The 5- and 7-year bFFF, CSS, and OS rates were 85.2 and 74.2%, 100 and 100%, and 95.7 and 91.9%, respectively. Only the iPSA ≤ 20 group was associated with the higher bFFF rate. The 7-year bFFF rates in the groups with iPSA ≤ 20 and iPSA > 20 were 86.6 and 48.6%, respectively.
Conclusion
HDR-BT plus EBRT without HT might be an alternative treatment option for patients with high-risk localized prostate cancer and iPSA levels ≤ 20. Further studies are required to validate the efficacy of this treatment strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is a malignant tumor that is becoming increasingly prevalent and is the most common cancer in men worldwide [1, 2]. Local radical treatment with surgery or radiotherapy is the recommended standard of care for patients with localized prostate cancer. The National Comprehensive Cancer Network (NCCN) guidelines (version 4, 2023) recommend combining hormone therapy (HT) with local radiotherapy for high-risk patients. In randomized trials of adding the HT to external beam radiotherapy (EBRT), the group that received long-term HT experienced improved local control and cause-specific survival (CSS) rate [3,4,5]. Ishiyama et al. conducted a multicenter, retrospective study on prostate cancer treatment using EBRT and high-dose-rate brachytherapy (HDR-BT). They found that the group receiving HT had significantly better outcomes in terms of biochemical control and clinical disease-free survival (DFS), as well as overall survival (OS) rates, than of those who did not receive it [6]. Biochemical freedom from failure (bFFF) was considerably superior in the HT addition group in a study on low-dose-rate brachytherapy (LDR-BT) in patients with prostate cancer in the intermediate-risk group [7]. However, previous studies have indicated that the addition of HT does not improve treatment outcomes. The reports on EBRT with LDR-BT for prostate cancer showed that adding HT did not improve bFFF, CSS, or OS [8, 9]. Furthermore, HT has several adverse effects and may not be routinely added depending on the patient’s general condition and willingness to receive the treatment [10]. Therefore, identifying a subgroup of patients in the high-risk prostate cancer group who receive HDR-BT and require less HT would be beneficial; however, there is a lack of sufficient evidence regarding this distinction. Since 1997, our institution has performed HDR-BT combined with EBRT for localized prostate cancer. However, HT was not introduced until 2019, with the exception of neoadjuvant HT. In this retrospective study, we aimed to examine the long-term outcomes in patients diagnosed with localized high-risk prostate cancer who received HDR-BT combined with EBRT without HT. Moreover, we aimed to identify the subgroup of patients who achieved superior results without requiring HT.
Materials and methods
Selection of patients
This study was approved by the Ethics Committee of Kawasaki Medical School, Okayama, Japan (approval number 5329-1). All procedures were performed in accordance with the ethical standards set forth in the Declaration of Helsinki and its subsequent amendments. A notification on the website offered the opportunity to withdraw from this study. We examined patients with prostate cancer who received HDR-BT and EBRT at our institution between April 1, 1997, and March 31, 2021, and who were available for follow-up for at least 6 months after treatment. Treatment techniques were identical to those previously reported [11].
Treatments
In this study, three-dimensional radiotherapy treatment planning in both HDR-BT and EBRT included the whole prostate as clinical target volume, but it excluded most of the seminal vesicles. HDR-BT was planned so that the minimum clinical target volume (CTV) dose was 95% of the prescribed dose. To comply with the organs at risk dose, the minimum CTV dose was allowed to be 90% of the prescribed dose. Urethra was allowed to be 120% of the prescribed dose and rectum 60% of the prescribed dose. EBRT was administered until March, 2009, using the four-field technique. Subsequently, three-dimensional conformal radiotherapy was initiated in April 2009 and intensity-modulated radiation therapy in April 2019. The HDR-BT and EBRT protocols were as follows: 16.5 Gy/3 fractions (Fr.) and 45 Gy/25 Fr. from April 1997 to March 1999, 22 Gy/4 Fr. and 45 Gy/25 Fr. from April 1999 to May 2000, 22 Gy/4 Fr. and 41.8 Gy/19 Fr. from June 2000 to December 2000, 24 Gy/4 Fr. and 36.8 Gy/16 Fr. from January 2001 to December 2006, and 20 Gy/2 Fr. (only 2, 18 Gy/2 Fr.) and 39 Gy/13 Fr. since January 2007. The treatment planning systems used for HDR-BT were PLATO (Nucletron, Veenendaal, Netherlands) and Oncentra version 3.3.86 (Nucletron) and version 4.5.3 (Nucletron). microSelectron version 2 (Nucletron) was used for treatment.
Inclusion criteria
The patients were categorized into five groups based on the NCCN guidelines (version 4, 2022), and the high-risk groups were identified. The high-risk group was characterized according to the presence of any one of the following factors: cT3a, less than five cores with grade group 4 or 5, and initial prostate-specific antigen (iPSA) level > 20 ng/mL.
The minimum patient age was 20 years with a general condition (ECOG performance status) ranging from 0 to 2. Magnetic resonance imaging and computed tomography (CT) were performed in all patients, and no metastases were observed. HT was not administered during the initial treatment phase. Patients were eligible for inclusion in this study regardless of whether they underwent pelvic lymph node dissection (PLND) or sampling. Patients with insufficient pathology findings to diagnose the grade group before treatment were excluded from this study. Biochemical failure was determined based on the Phoenix definition [12].
Statistical considerations
The bFFF, CSS, and OS rates were calculated. The bFFF is the biochemical relapse-free rate and is often used in prostate cancer analysis [7]. Grade 5 case was categorized as a prostate cancer-related fatality. The bFFF, CSS, and OS rates were analyzed based on the clinical T stage, biologically effective dose (BED), pathological grade, PLND, PSA level, and age. BED was calculated as follows: α/β = 1.5 [13]. Variables with p-values < 0.1 in the univariate analysis were further analyzed using the Cox proportional hazards model in a multivariate analysis. Statistical significance was defined as a p-value of < 0.05. Statistical analyses were performed using the SPSS version 20 software (SPSS Inc. Chicago, IL).
Toxicity
The adverse events of genitourinary and gastrointestinal toxicities were assessed using the Common Terminology Criteria for Adverse Events version 5.0.
Results
Clinical characteristics
Patient characteristics are shown in Table 1. In all, 72 patients were treated, with a median observation period of 91.9 (range, 15.1–189.1) months. There was one case in which the patient did not receive the addition of HT after April 2019 at the patient’s own request. The median age and iPSA level were 71 (range, 52–81) years and 10.95 (range, 4.3–139.0) ng/mL, respectively. All patients had adenocarcinoma, and the grade groups were 1 for 14, 2 for 18, 3 for 8, 4 for 21, and 5 for 11 patients. In total, 9, 23, 13, 9, and 18 patients had T1c, T2a, T2b, T2c, and T3a stages, respectively. Forty-three patients underwent PLND. The four-field technique was performed in 42 patients, three-dimensional conformal radiotherapy in 29, and intensity-modulated radiation therapy in 1. Details of the HDR-BT combined with EBRT protocols are presented in Table 2. The median BED for HDR-BT combined with EBRT was 270.3 (range, 176.0–270.3) Gy. Biochemical failure was observed in 21 patients. The median time to biochemical failure was 60.03 (range, 7–171.43) months. Eleven patients died during the observation period. In all patients, HT was initiated after biochemical recurrence. The first site of clinical recurrence was bone metastasis in five patients, two were in the iPSA ≤ 20 group and three in iPSA > 20 group. There were two deaths due to prostate cancer, both in the high-dose group, one of which was classified as grade 5. There were nine deaths from other diseases with no metastases, and biochemical failure was seen in three.
Oncological endpoints
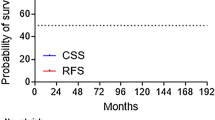
The 5- and 7-year bFFF rates were 85.2 and 74.2%, respectively (Fig. 1a). Univariate analysis was conducted for bFFF, CSS, and OS by dividing each factor into two groups. Age and BED were divided into ≥ 71 and < 71 median years and ≥ 270.3 and < 270.3 median Gy, respectively. iPSA, grade group, and clinical T stage were divided into high-risk group factors: > 20 ng/ml vs ≤ 20 ng/ml, ≥ 4 vs ≤ 3, and cT3a vs ≤ cT2. PLND was divided into two groups: with and without PLND. In the univariate analysis, the iPSA ≤ 20 and higher-grade groups were associated with the high bFFF group (p < 0.001 and p = 0.023, respectively). In the multivariate analysis, only the iPSA ≤ 20 group was associated with the high bFFF group (p = 0.024; Table 3). Details of the iPSA ≤ 20 and iPSA > 20 groups are shown in Supplemental Table 1. The 7-year bFFF rates in the groups with iPSA ≤ 20 and iPSA > 20 were 86.6 and 48.6%, respectively (Fig. 2). Of the five patients with distant metastasis after biochemical recurrence, two were in the iPSA ≤ 20 group and three in iPSA > 20 group. The 5- and 7-year CSS rates were 100 and 100%, respectively (Fig. 1b). In the univariate analysis, a lower BED was associated with a higher CSS rate (p = 0.043). The results of the CSS analysis are shown in Supplemental Table 2. The 5- and 7-year OS rates were 95.7 and 91.9%, respectively (Fig. 1c). Univariate analysis revealed no significant factors affecting OS. There were no significant differences in the CSS and OS between the groups with and without biochemical failure (p = 0.093 and 0.792, respectively).
a Biochemical freedom from failure rate. The 5- and 7-year biochemical freedom from failure rates are 85.2 and 74.2%, respectively. b Cause-specific survival rate. The 5- and 7-year cause-specific survival rates are 100 and 100%, respectively. c Overall survival rate. The 5- and 7-year overall survival rates are 95.7 and 91.9%, respectively
Acute and late adverse events
No acute adverse events of grade 3 or higher were observed. Late adverse events included grade 3 urethral stricture in six patients, proctitis in one patient, and grade 5 bladder bleeding in one patient. Biochemical failure occurred 3 years and 6 months after treatment, and HT was initiated in a patient with grade 5 disease. Intermittent hematuria was observed 5 years after treatment, followed by refractory hematuria. Subsequently, he died 7 years and 1 month after the treatment.
Discussion
Our study demonstrated that HDR-BT in combination with EBRT without additional HT resulted in 7-year bFFF, CSS, and OS rates of 74.2, 100, and 91.9%, respectively, indicating that iPSA was a significant prognostic factor for bFFF. The addition of HT to radiotherapy for high-risk patients with localized prostate cancer is standard of care and is intended to improve bFFF and survival [5, 14,15,16]. In these trials, radiotherapy was administered at doses as low as 70 Gy. Radiotherapy improves the rate of biochemical control in patients with prostate cancer by escalating the radiation dose, and the combination of brachytherapy and EBRT is also utilized to increase the dose to the prostate with minimal risk to neighboring organs. Ishiyama et al. conducted a large study of 3,424 patients treated with HDR-BT for prostate cancer and found that in the high-risk group, treatment of prostate cancer with the addition of HT resulted in better biochemical control, clinical DFS, and OS than that without additional HT [6]. Since 2019, when this paper was published, we have standardized the addition of HT to groups that are more advanced than the unfavorable intermediate-risk group at our institution.
The 8-year bFFF, CSS, and OS rates of 156 high-risk patients treated with HDR-BT in combination with EBRT without HT were 53.9, 95, and 76.1%, respectively [17]. Similarly, Prada et al. reported a 10-year bFFF rate of 74% [18]. For a study on LDR-BT without additional HT, Stone et al. reported that the high-risk group with GS ≥ 7 had a 5-year bFFF rate of 77.5% [19]. In a study of EBRT alone without additional HT, Krauss et al. reported 5-year bFFF and OS rates of 72.2 and 76.2%, respectively, in a high-risk group of 29 patients [17]. Our study results showed that the bFFF rate was comparable to those from other studies. The CSS and OS rates in our study were better than those reported previously. In our patients experiencing PSA recurrence, HT was immediately initiated in our study. If immediate administration of HT after PSA recurrence results in favorable CSS and OS, then a treatment strategy of HDR-BT in combination with EBRT, followed by deferral of HT, may be an alternative treatment for patients who do not wish to receive HT or are concerned about its adverse effects. Regarding CSS in our study, two patients in the higher BED group died of prostate cancer, resulting in a lower CSS in the higher BED group than in the lower BED group. In HDR-BT in combination with EBRT, there is a study reporting an improved CSS with higher doses, and our result differs from theirs [20]. Rationalizing our findings regarding prognostic factors for CSS is challenging. This detection may have incidentally occurred because of the limited number of cases or may have been affected by the inclusion of a grade 5 patient’s registration as a prostate cancer death.
In our study, the iPSA level was a significant prognostic factor for bFFF. Previous reports have shown improved outcomes in patients with iPSA levels ≤ 20 ng/mL. Krauss et al. reported that iPSA was a predictor of biochemical failure in all risk groups treated with EBRT alone or HDR-BT in combination with EBRT, with or without HT [17]. Martinez et al. also reported that iPSA and the Gleason score influenced biochemical failure in the treatment of HDR-BT in combination with EBRT and HT in high-risk groups [20]. Stone et al. administered LDR-BT in combination with EBRT without HT to patients with a Gleason score of ≥ 7 and found that the group with iPSA levels ≤ 20 ng/mL had improved bFFF [19]. Higher levels of PSA indicate a greater probability of detecting further lesions outside the prostate [21, 22]. Recent investigations using prostate-specific membrane antigen positron emission tomography (PSMA-PET) have also shown a positive correlation between PSA levels and the degree of extra-prostatic lesion accumulation [23, 24]. PSMA-PET has a superior detection rate compared with CT or bone scintigraphy for identifying extra-prostatic lesions in high-risk groups [25]. Additionally, Leeuwen et al. reported higher PSMA accumulation in patient post-prostatectomy with high PSA levels than in those not detected on CT or bone scintigraphy [26]. Biochemical recurrence was more prevalent in patients with iPSA levels > 20 ng/mL than in those with iPSA levels ≤ 20 ng/mL, and although PSMA-PET was not used in our study, it is reasonable to hypothesize the presence of extra-prostatic lesions. Our results showed a favorable 7-year bFFF rate of 86.6% in the iPSA ≤ 20 group, and HDR-BT in combination with EBRT without additional HT may be a promising alternative treatment for patients with iPSA levels ≤ 20 ng/mL.
In general, HT is associated with several adverse effects, such as sexual dysfunction, osteoporosis, hot flashes, worsening of metabolic disorders, fatigue, gynecomastia, reduction in penis and testicular size, weight gain, thinning hair, increased risk of cognitive decline, and exacerbation of cardiovascular disease and diabetes [27,28,29,30,31]. Yamazaki et al. found that prolonged HT for > 2 years might increase the possibility of mortality from non-prostate cancer causes [32]. A comparison of the outcomes of the short-term and long-term HT groups in the 10-year follow-up report of the DART trial demonstrated no statistically significant differences in biochemical DFS or OS in the high-risk group [33]. In the current age of high-dose radiation techniques, such as intensity-modulated radiotherapy and HDR-BT, the usefulness of HT may be less significant compared with that in the past, emphasizing the need for additional evidence.
Grade 5 bladder bleeding was observed in one patient. A study of 709 patients treated with radiotherapy suggested that the prevalence of hemorrhagic cystitis was not higher in patients who underwent brachytherapy [34]. Four patients (0.7%) had grade 5 hemorrhagic cystitis, but the study failed to specify the radiation technique that contributed to this event. In the grade 5 patient in our study, radiation doses were not markedly higher than those previously reported, and the underlying reason was not apparent, considering the patient background. Therefore, adequate informed consent and long-term follow-up are preferable.
Our study is limited by the small patient population at a single facility, the absence of standardized doses and fractions, and the potential benefit of excluding the very high-risk group classified by the NCCN. Ishiyama et al. reported a 10-year bFFF of 74.7% in their group without HT, compared with an improved 10-year bFFF of 83.0% in their group with HT in all risk groups [6]. Although the classification of high-risk groups in Yorozu et al.’s investigation differs from the one in our study, they reported a 7-year bFFF of 92% with the addition of HT in their study of LDR-BT [35]. In our study, the 7-year bFFF was 74.2%, a poor result compared to these reports with the addition of HT. Treatment without additional HT should be performed with informed consent. Nevertheless, our study is important because it demonstrated long-term outcomes, namely, a 7-year bFFF rate of 86.6% in the subgroup with iPSA ≤ 20.
In conclusion, we report the long-term outcomes of HDR-BT in combination with EBRT without additional HT for high-risk localized prostate cancer. Considering that the addition of HT is the standard of care for the treatment of high-risk localized prostate cancer, HDR-BT in combination with EBRT without the addition of HT might be an alternative treatment option for patients with iPSA ≤ 20 levels.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. https://doi.org/10.3322/caac.21262.
Rebbeck TR, Haas GP. Temporal trends and racial disparities in global prostate cancer prevalence. Can J Urol. 2014;21:7496–506.
Denham JW, Steigler A, Lamb DS, Joseph D, Mameghan H, Turner S, et al. Short-term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans-Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841–50. https://doi.org/10.1016/S1470-2045(05)70348-X.
See WA, Tyrrell CJ, CASODEX Early Prostate Cancer Trialist’s Group. The addition of bicalutamide 150 mg to radiotherapy significantly improves overall survival in men with locally advanced prostate cancer. J Cancer Res Clin Oncol. 2006;132(Suppl 1):S7-SS16. https://doi.org/10.1007/s00432-006-0132-6.
Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. https://doi.org/10.1056/NEJMoa0810095.
Ishiyama H, Kamitani N, Kawamura H, Kato S, Aoki M, Kariya S, et al. Nationwide multi-institutional retrospective analysis of high-dose-rate brachytherapy combined with external beam radiotherapy for localized prostate cancer: an Asian Prostate HDR-BT Consortium. Brachytherapy. 2017;16:503–10. https://doi.org/10.1016/j.brachy.2017.01.006.
Katayama N, Nakamura K, Yorozu A, Kikuchi T, Fukushima M, Saito S, et al. Biochemical outcomes and predictive factors by risk group after permanent iodine-125 seed implantation: prospective cohort study in 2,316 patients. Brachytherapy. 2019;18:574–82. https://doi.org/10.1016/j.brachy.2019.03.008.
Stock RG, Yalamanchi S, Hall SJ, Stone NN. Impact of hormonal therapy on intermediate risk prostate cancer treated with combination brachytherapy and external beam irradiation. J Urol. 2010;183:546–50. https://doi.org/10.1016/j.juro.2009.10.006.
Merrick GS, Butler WM, Galbreath RW, Lief J, Bittner N, Wallner KE, et al. Prostate cancer death is unlikely in high-risk patients following quality permanent interstitial brachytherapy. BJU Int. 2011;107:226–32. https://doi.org/10.1111/j.1464-410X.2010.09486.x.
Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. https://doi.org/10.1093/jnci/djp404.
Hiratsuka J, Jo Y, Yoshida K, Nagase N, Fujisawa M, Imajo Y. Clinical results of combined treatment conformal high-dose-rate iridium-192 brachytherapy and external beam radiotherapy using staging lymphadenectomy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2004;59:684–90. https://doi.org/10.1016/j.ijrobp.2003.11.035.
Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–74. https://doi.org/10.1016/j.ijrobp.2006.04.029.
Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys Biol Phys. 2012;82:17–24. https://doi.org/10.1016/j.ijrobp.2010.10.075.
Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–73. https://doi.org/10.1016/S1470-2045(10)70223-0.
Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285–90. https://doi.org/10.1016/j.ijrobp.2004.08.047.
D’Amico AV, Moran BJ, Braccioforte MH, Dosoretz D, Salenius S, Katin M, et al. Risk of death from prostate cancer after brachytherapy alone or with radiation, androgen suppression therapy, or both in men with high-risk disease. J Clin Oncol. 2009;27:3923–8. https://doi.org/10.1200/JCO.2008.20.3992.
Krauss D, Kestin L, Ye H, Brabbins D, Ghilezan M, Gustafson G, et al. Lack of benefit for the addition of androgen deprivation therapy to dose-escalated radiotherapy in the treatment of intermediate- and high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2011;80:1064–71. https://doi.org/10.1016/j.ijrobp.2010.04.004.
Prada PJ, Mendez L, Fernández J, González H, Jiménez I, Arrojo E. Long-term biochemical results after high-dose-rate intensity modulated brachytherapy with external beam radiotherapy for high risk prostate cancer. Radiat Oncol. 2012;7:31. https://doi.org/10.1186/1748-717X-7-31.
Stone NN, Potters L, Davis BJ, Ciezki JP, Zelefsky MJ, Roach M, et al. Multicenter analysis of effect of high biologic effective dose on biochemical failure and survival outcomes in patients with Gleason score 7–10 prostate cancer treated with permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2009;73:341–6. https://doi.org/10.1016/j.ijrobp.2008.04.038.
Martinez AA, Gonzalez J, Ye H, Ghilezan M, Shetty S, Kernen K, et al. Dose escalation improves cancer-related events at 10 years for intermediate- and high-risk prostate cancer patients treated with hypofractionated high-dose-rate boost and external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:363–70. https://doi.org/10.1016/j.ijrobp.2009.10.035.
Lee N, Newhouse JH, Olsson CA, Benson MC, Petrylak DP, Schiff PB, et al. Which patients with newly diagnosed prostate cancer need a computed tomography scan of the abdomen and pelvis? An analysis based on 588 patients. Urology. 1999;54:490–4. https://doi.org/10.1016/s0090-4295(99)00150-8.
Briganti A, Passoni N, Ferrari M, Capitanio U, Suardi N, Gallina A, et al. When to perform bone scan in patients with newly diagnosed prostate cancer: external validation of the currently available guidelines and proposal of a novel risk stratification tool. Eur Urol. 2010;57:551–8. https://doi.org/10.1016/j.eururo.2009.12.023.
Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–37. https://doi.org/10.1016/j.eururo.2016.06.021.
Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68GA: PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–68. https://doi.org/10.1007/s00259-017-3711-7.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/S0140-6736(20)30314-7.
van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–9. https://doi.org/10.1111/bju.13397.
Gay HA, Sanda MG, Liu J, Wu N, Hamstra DA, Wei JT, et al. External beam radiation therapy or brachytherapy with or without short-course neoadjuvant androgen deprivation therapy: results of a multicenter, prospective study of quality of life. Int J Radiat Oncol Biol Phys. 2017;98:304–17. https://doi.org/10.1016/j.ijrobp.2017.02.019.
Nguyen PL, Alibhai SM, Basaria S, D’Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67:825–36. https://doi.org/10.1016/j.eururo.2014.07.010.
Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–76. https://doi.org/10.1200/JCO.2008.18.4184.
Stone P, Hardy J, Huddart R, A’Hern R, Richards M. Fatigue in patients with prostate cancer receiving hormone therapy. Eur J Cancer. 2000;36:1134–41. https://doi.org/10.1016/s0959-8049(00)00084-8.
Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–56. https://doi.org/10.1200/JCO.2006.06.2497.
Yamazaki H, Masui K, Suzuki G, Nakamura S, Aibe N, Shimizu D, et al. Effect of androgen deprivation therapy on other-cause of mortality in elderly patients with clinically localized prostate cancer treated with modern radiotherapy: is there a negative impact? J Clin Med. 2019. https://doi.org/10.3390/jcm8030338.
Zapatero A, Guerrero A, Maldonado X, Álvarez A, San-Segundo CG, Rodríguez MÁC, et al. High-dose radiotherapy and risk-adapted androgen deprivation in localised prostate cancer (DART 01/05): 10-year results of a phase 3 randomised, controlled trial. Lancet Oncol. 2022;23:671–81. https://doi.org/10.1016/S1470-2045(22)00190-5.
Martin SE, Begun EM, Samir E, Azaiza MT, Allegro S, Abdelhady M. Incidence and morbidity of radiation-induced hemorrhagic cystitis in prostate cancer. Urology. 2019;131:190–5. https://doi.org/10.1016/j.urology.2019.05.034.
Yorozu A. Trimodality therapy with Iodine-125 brachytherapy, external beam radiation therapy, and short- or long-term androgen deprivation therapy for high-risk localized prostate cancer: results of a multicenter, randomized phase 3 trial (TRIP/TRIGU0907). Int J Radiat Oncol Biol Phys. 2024;118:390–401. https://doi.org/10.1016/j.ijrobp.2023.08.046.
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
NK collected the data, performed data analysis, and drafted the manuscript. KW supervised this study. KK drafted the manuscript and supervised this study. All the authors participated in the design of this study and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Ethics Committee of Kawasaki Medical School (approval number: 5329-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kamitani, N., Watanabe, K., Ikeda, N. et al. Long-term outcomes of high-dose-rate brachytherapy and external beam radiotherapy without hormone therapy for high-risk localized prostate cancer. Jpn J Radiol (2024). https://doi.org/10.1007/s11604-024-01621-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11604-024-01621-4