Abstract
Purpose
Predicting progression of mild cognitive impairment (MCI) to Alzheimer’s disease (AD) or dementia with Lewy bodies (DLB) is important. We evaluated morphological and functional differences between MCI with Lewy bodies (MCI-LB) and MCI due to AD (MCI-AD), and a method for differentiating between these conditions using brain MRI and brain perfusion SPECT.
Methods
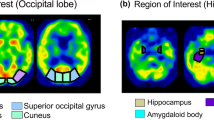
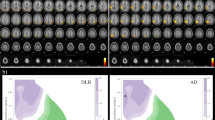
A continuous series of 101 subjects, who had visited our memory clinic and met the definition of MCI, were enrolled retrospectively. They were consisted of 60 MCI-LB and 41 MCI-AD subjects. Relative cerebral blood flow (rCBF) on SPECT images and relative brain atrophy on MRI images were evaluated. We performed voxel-based analysis and visually inspected brain perfusion SPECT images for regional brain atrophy, occipital hypoperfusion and the cingulate island sign (CIS), for differential diagnosis of MCI-LB and MCI-AD.
Results
MRI showed no significant differences in regional atrophy between the MCI-LB and MCI-AD groups. In MCI-LB subjects, occipital rCBF was significantly decreased compared with MCI-AD subjects (p < 0.01, family wise error [FWE]-corrected). Visual inspection of occipital hypoperfusion had sensitivity, specificity, and accuracy values of 100%, 73.2% and 89.1%, respectively, for differentiating MCI-LB and MCI-AD. Occipital hypoperfusion was offered higher diagnostic utility than the CIS.
Conclusions
The occipital lobe was the region with significantly decreased rCBF in MCI-LB compared with MCI-AD subjects. Occipital hypoperfusion on brain perfusion SPECT may be a more useful imaging biomarker than the CIS for visually differentiating MCI-LB and MCI-AD.




Similar content being viewed by others
References
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8.
Ikejima C, Hisanaga A, Meguro K, Yamada T, Ouma S, Kawamuro Y, et al. Multicentre population-based dementia prevalence survey in Japan: a preliminary report. Psychogeriatrics. 2012;12:120–3.
Frisoni GB, Fratiglioni L, Fastbom J, Guo Z, Viitanen M, Winblad B. Mild cognitive impairment in the population and physical health: data on 1,435 individuals aged 75 to 95. J Gerontol A Biol Sci Med Sci. 2000;55:M322–8.
Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia–meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–65.
Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–72.
Kishi T, Matsunaga S, Oya K, Ikuta T, Iwata N. Protection against brain atrophy by anti-dementia medication in mild cognitive impairment and Alzheimer’s disease: meta-analysis of longitudinal randomized placebo-controlled trials. Int J Neuropsychopharmacol. 2015. https://doi.org/10.1093/ijnp/pyv070.
Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: a systematic review and meta-analysis. CMAJ. 2013;185:1393–401.
Sugimoto T, Sakurai T, Akatsu H, Doi T, Fujiwara Y, Hirakawa A, et al. The Japan-Multimodal Intervention Trial for Prevention of Dementia (J-MINT): the study protocol for an 18-month, multicenter, randomized, controlled trial. J Prev Alzheimers Dis. 2021. https://doi.org/10.14283/jpad.2021.29.
Shimada H, Makizako H, Doi T, Park H, Tsutsumimoto K, Verghese J, et al. Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J Am Med Dir Assoc. 2018;19:584–91.
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology. 2018;90:126–35.
Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–734.
Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Ito K, et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE. 2013;8: e61483.
Brodaty H, Heffernan M, Kochan NA, Draper B, Trollor JN, Reppermund S, et al. Mild cognitive impairment in a community sample: the Sydney Memory and Ageing Study. Alzheimers Dement. 2013;9:310-7.e1.
Suzuki T, Shimada H, Makizako H, Doi T, Yoshida D, Tsutsumimoto K, et al. Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. 2012;12:128.
Lautenschlager NT, Cox K, Kurz AF. Physical activity and mild cognitive impairment and Alzheimer’s disease. Curr Neurol Neurosci Rep. 2010;10:352–8.
Yokoi K, Nishio Y, Uchiyama M, Shimomura T, Iizuka O, Mori E. Hallucinators find meaning in noises: pareidolic illusions in dementia with Lewy bodies. Neuropsychologia. 2014;56:245–54.
Swanson CJ, Zhang Y, Dhadda S, Wang J, Kaplow J, Lai RYK, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13:80.
Salloway S, Chalkias S, Barkhof F, Burkett P, Barakos J, Purcell D, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79:13–21.
Matsuda H, Yokoyama K, Sato N, Ito K, Nemoto K, Oba H, et al. Differentiation between dementia with Lewy bodies And Alzheimer’s disease using voxel-based morphometry of structural MRI: a multicenter study. Neuropsychiatr Dis Treat. 2019;15:2715–22.
Goto H, Ishii K, Uemura T, Miyamoto N, Yoshikawa T, Shimada K, et al. Differential diagnosis of dementia with Lewy Bodies and Alzheimer Disease using combined MR imaging and brain perfusion single-photon emission tomography. AJNR Am J Neuroradiol. 2010;31:720–5.
Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130:708–19.
Middelkoop HA, van der Flier WM, Burton EJ, Lloyd AJ, Paling S, Barber R, et al. Dementia with Lewy bodies and AD are not associated with occipital lobe atrophy on MRI. Neurology. 2001;57:2117–20.
Barber R, Gholkar A, Scheltens P, Ballard C, McKeith IG, O’Brien JT. Medial temporal lobe atrophy on MRI in dementia with Lewy bodies. Neurology. 1999;52:1153–8.
O’Brien JT, Firbank MJ, Davison C, Barnett N, Bamford C, Donaldson C, et al. 18F-FDG PET and perfusion SPECT in the diagnosis of Alzheimer and Lewy body dementias. J Nucl Med. 2014;55:1959–65.
Chiba Y, Iseki E, Fujishiro H, Ota K, Kasanuki K, Suzuki M, et al. Early differential diagnosis between Alzheimer’s disease and dementia with Lewy bodies: comparison between (18)F-FDG PET and (123)I-IMP SPECT. Psychiatry Res Neuroimaging. 2016;249:105–12.
Massa F, Chincarini A, Bauckneht M, Raffa S, Peira E, Arnaldi D, et al. Added value of semiquantitative analysis of brain FDG-PET for the differentiation between MCI-Lewy bodies and MCI due to Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2022;49:1263–74.
Arbizu J, Festari C, Altomare D, Walker Z, Bouwman F, Rivolta J, et al. Clinical utility of FDG-PET for the clinical diagnosis in MCI. Eur J Nucl Med Mol Imaging. 2018;45:1497–508.
Chiba Y, Fujishiro H, Iseki E, Kasanuki K, Sato K. The Cingulate Island Sign on FDG-PET vs. IMP-SPECT to assess mild cognitive impairment in Alzheimer’s disease vs. Dementia with Lewy Bodies. J Neuroimaging. 2019;29:712–20.
Roberts G, Durcan R, Donaghy PC, Lawley S, Ciafone J, Hamilton CA, et al. Accuracy of cardiac innervation scintigraphy for mild cognitive impairment with Lewy bodies. Neurology. 2021;96:e2801–11.
Thomas AJ, Donaghy P, Roberts G, Colloby SJ, Barnett NA, Petrides G, et al. Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med. 2019;49:396–402.
McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94:743–55.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
Nakata T, Shimada K, Iba A, Oda H, Terashima A, Koide Y, et al. Correlation between noise pareidolia test scores for visual hallucinations and regional cerebral blood flow in dementia with Lewy bodies. Ann Nucl Med. 2022;36:384–92.
Ishii K. Diagnostic imaging of dementia with Lewy bodies, frontotemporal lobar degeneration, and normal pressure hydrocephalus. Jpn J Radiol. 2020;38:64–76.
Walker Z, Jaros E, Walker RW, Lee L, Costa DC, Livingston G, et al. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J Neurol Neurosurg Psychiatry. 2007;78:1176–81.
Tatsch K, Poepperl G. Nigrostriatal dopamine terminal imaging with dopamine transporter SPECT: an update. J Nucl Med. 2013;54:1331–8.
Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113.
Ishii K, Soma T, Shimada K, Oda H, Terashima A, Kawasaki R. Automatic volumetry of the cerebrospinal fluid space in idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Dis Extra. 2013;3:489–96.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100.
Imabayashi E, Yokoyama K, Tsukamoto T, Sone D, Sumida K, Kimura Y, et al. The cingulate island sign within early Alzheimer’s disease-specific hypoperfusion volumes of interest is useful for differentiating Alzheimer’s disease from dementia with Lewy bodies. EJNMMI Res. 2016;6:67.
Graff-Radford J, Murray ME, Lowe VJ, Boeve BF, Ferman TJ, Przybelski SA, et al. Dementia with Lewy bodies: basis of cingulate island sign. Neurology. 2014;83:801–9.
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–48.
Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50:1638–45.
Imamura T, Ishii K, Sasaki M, Kitagaki H, Yamaji S, Hirono N. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease: a comparative study using positron emission tomography. Neurosci Lett. 1997;235:49–52.
Tilley BS, Patel SR, Goldfinger MH, Pearce RKB, Gentleman SM. Locus coeruleus pathology indicates a continuum of Lewy Body dementia. J Parkinsons Dis. 2021;11:1641–50.
Kantarci K, Boeve BF, Przybelski SA, Lesnick TG, Chen Q, Fields J, et al. FDG PET metabolic signatures distinguishing prodromal DLB and prodromal AD. Neuroimage Clin. 2021;31: 102754.
Zhu L, Zhao W, Chen J, Li G, Qu J. Systematic review and meta-analysis of diagnostic test accuracy (DTA) studies: the role of cerebral perfusion imaging in prognosis evaluation of mild cognitive impairment. Ann Palliat Med. 2022;11:673–83.
Kanetaka H, Shimizu S, Inagawa Y, Hirose D, Takenoshita N, Sakurai H, et al. Differentiating mild cognitive impairment, Alzheimer’s disease, and dementia with Lewy bodies using cingulate island sign on perfusion IMP-SPECT. Front Neurol. 2020;11:568438.
Roquet D, Noblet V, Anthony P, Philippi N, Demuynck C, Cretin B, et al. Insular atrophy at the prodromal stage of dementia with Lewy bodies: a VBM DARTEL study. Sci Rep. 2017;7:9437.
Blanc F, Colloby SJ, Philippi N, de Petigny X, Jung B, Demuynck C, et al. Cortical thickness in dementia with Lewy Bodies and Alzheimer’s disease: a comparison of prodromal and dementia stages. PLoS ONE. 2015;10:e0127396.
Firbank MJ, O’Brien JT, Durcan R, Allan LM, Barker S, Ciafone J, et al. Mild cognitive impairment with Lewy bodies: blood perfusion with arterial spin labelling. J Neurol. 2021;268:1284–94.
Duan W, Zhou GD, Balachandrasekaran A, Bhumkar AB, Boraste PB, Becker JT, et al. Cerebral blood flow predicts conversion of mild cognitive impairment into Alzheimer’s disease and cognitive decline: an arterial spin labeling follow-up study. J Alzheimers Dis. 2021;82:293–305.
Ishii K, Yamaji S, Kitagaki H, Imamura T, Hirono N, Mori E. Regional cerebral blood flow difference between dementia with Lewy bodies and AD. Neurology. 1999;53:413–6.
Catafau AM, Tolosa E. Impact of dopamine transporter SPECT using 123I-Ioflupane on diagnosis and management of patients with clinically uncertain Parkinsonian syndromes. Mov Disord. 2004;19:1175–82.
Funding
This study received no funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Informed consent
All investigations were carried out according to the Declaration of Helsinki. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. All co-authors have read and approved the submission. This study was approved by the ethics committee of Hyogo Brain and Heart Center (now called Hyogo Prefectural Harima-Himeji General Medical Center) and the requirement to obtain written informed consent was waived because this was a retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nakata, T., Shimada, K., Iba, A. et al. Differential diagnosis of MCI with Lewy bodies and MCI due to Alzheimer’s disease by visual assessment of occipital hypoperfusion on SPECT images. Jpn J Radiol 42, 308–318 (2024). https://doi.org/10.1007/s11604-023-01501-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-023-01501-3