Abstract
Numerous studies have clarified the usefulness of 18F-fluorodeoxyglucose (FDG)-PET/CT (positron emission tomography) for diagnosing the cause of fever of unknown origin (FUO). Various types of disease can cause FUO, but the cause remains unknown in a certain proportion of FUO, even when the advanced diagnostic methodologies are used. FDG-PET/CT is regarded as a second-line modality in the diagnostic process of FUO, and its potential to identify the cause of FUO will be maximized when the appropriate clinical considerations are understood. Accordingly, this review presents basic knowledge regarding FUO, and reports the current status of FDG-PET/CT applied to diagnosing the cause of FUO, including diagnostic performance, test protocols, possible factors influencing the diagnostic result, outcomes, and cost-effectiveness. This knowledge will enable effective future use of FDG-PET/CT to improve outcomes in patients with FUO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1961, Petersdorf and Beeson defined fever of unknown origin (FUO) as "a condition in which fever of 38.3 °C or higher as sublingual temperature is observed several times for more than 3 weeks, and the cause is unknown even after hospitalization for 1 week or longer" [1]. In 1991, Durack and Street revised the criteria as "a condition in which fever of 38.3 °C from unknown origin persists for more than 3 weeks and cannot be diagnosed by 3 days of inpatient examination or 3 outpatient examinations” [2]. FUO based on these two criteria is termed “classic FUO”. FUO is a common manifestation of multiple disparate disease processes that can be subcategorized into classic FUO, nosocomial FUO (FUO that develops in hospitalized persons), immunodeficiency-associated FUO, and travel-associated FUO. More recently, FUO has been defined as (1) fever ≥ 38.3 °C (≥ 101 ℉) on at least two occasions, (2) illness duration of ≥ 3 weeks, (3) no known immunocompromised state, (4) uncertain diagnosis even after thorough history-taking, physical examination, and the following obligatory investigations: determination of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level, platelet count, leukocyte count and differential, measurement of levels of hemoglobin (Hb), electrolytes, creatinine, total protein, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, creatine kinase, ferritin, antinuclear antibodies, and rheumatoid factor; protein electrophoresis, urinalysis, blood cultures, urine culture, and chest X-ray; abdominal ultrasonography (AUS), and tuberculin skin test or interferon γ release assay [3]. As clinical studies of FDG-PET or FDG-PET/CT for the evaluation of FUO have mostly employed the definition of classic FUO using either the Petersdorf and Beeson or Durack and Street criteria, this review therefore focuses on classic FUO.
The majority of diseases that cause FUO are well known and are discussed later in this review. Although evidence-based diagnostic criteria and guidelines have been established for these diseases, FUO describes the state in which the background disease cannot be identified by the diagnostic workup. In general, unknown cause of prolonged fever leads to limitations in daily life that include various physical symptoms, mental load, and total debilitation that may be related to social and economic losses. Considering the known severity and prognosis of the various diseases that cause FUO, irreversible disorders or death can occur if left untreated.
When the cause of FUO is not identified through the diagnostic workup, FDG-PET/CT can be a gamechanger for guiding a final diagnosis. However, it should be used efficiently in the clinical situation due to its limitations of radiation exposure and the high cost.
Mechanism of fever
Fever is an active increase in body temperature and is regulated by the hypothalamus. When a bacterium or virus invades the body, it is recognized by Toll-like receptors (TLRs) present in macrophage cells and dendritic cells. When TLRs are stimulated, inflammatory cytokines such as interleukin (IL)-1β and IL-6 are released, which stimulate the production of acute phase proteins such as CRP, fibrinogen, and serum amyloid A protein. When inflammatory cytokines produced in peripheral tissues act on cytokine receptors in cerebrovascular endothelial cells, they promote the production of prostaglandin E2 (PGE2) which is a mediator of fever. When PGE2 acts on the EP3 receptor in the preoptic area (POA), which is the center of thermoregulation, the thermogenic reaction is enhanced and the heat dissipation reaction is suppressed [4,5,6,7,8].
In addition to cerebrovascular endothelial cells, fever is also caused by PGE2 produced by macrophages in the lungs and liver, afferent signals from vagus nerves and somatosensory nerves located locally in inflammation, and activation of microglia and astrocytes caused by peripheral inflammation. Non-shivering heat production and cutaneous vasoconstriction in brown adipose tissue, which play major roles in the exothermic reaction, are caused by increased sympathetic nerve activity. The sympathetic nerves involved in fever are the dorsomedial hypothalamus (DMH), the rostral ventromedial part of the medulla oblongata centered on the rostral nucleus raphe plaque and the large raphe nucleus, and the thoracic spinal cord to the upper lumbar spinal cord, due to excitement of neurons at the level of the intermediolateral nucleus (IML) of the spinal cord [5]. Neurons with persistent inhibitory control project from the POA to the DMH and the ventromedial part of the medulla oblongata, but when PGE2 acts on the EP3 receptor of POA, the activity of POA neurons decreases. Thus, fever is caused by release of the brake of the POA on the DMH and the sympathetic nervous system by PGE2 [4]. Fever can be caused not only by infectious diseases but also by autoimmune diseases, malignant tumors, tissue disorders, such as surgical invasion and myocardial infarction, and cytokine therapy related to PGE2 activity [9]. Abundant inflammatory cells such as neutrophils and macrophages can exist in an area that is the cause of fever, and can thus be identified by FDG-PET/CT as accumulation in these inflammatory cells.
Possible responsible causative diseases of FUO and their trends
FUO can be caused by a wide group of diseases, including both benign and serious conditions. The main categories of diseases responsible for FUO are infections, noninfectious inflammatory disease (NIID), neoplasm (mainly malignancy), and miscellaneous others. Among bacterial infections, tuberculosis is the most common infectious cause of FUO [10], and others include infective endocarditis, abscess, prostatitis, Whipple’s disease, and typhoidal and nontyphoidal salmonella serovars [3]. Viral infections that can cause FUO include cytomegalovirus, Epstein–Barr virus (EBV), human herpesvirus (HHV)-6, and HHV-7 [11]. EBV viremia is characterized by fever with hematologic abnormalities. The clinical presentation of infectious mononucleosis can vary with age. HHV-6 and HHV-8 should generally be tested only in immunocompromised patients [12]. Fungal infections, such as aspergillosis and cryptococcosis, tend to occur in immunocompromised persons [10]. Neoplasms commonly associated with FUO, based on pyrogenic cytokine production or spontaneous tumor necrosis, include lymphomas, renal-cell carcinoma, hepatocellular and ovarian cancer, atrial myxoma, and Castleman’s disease [8, 13]. Causative NIIDs consist mainly of autoinflammatory and autoimmune disorders, alone or in combination. Autoinflammatory conditions are disorders of innate immunity with dysregulated interleukin-1β responses, interleukin-18 responses, or both. Autoimmune diseases involve adaptive immunity and are driven by a type 1 interferon response [14]. Adult-onset Still’s disease (AOSD) and rheumatoid arthritis (RA), giant-cell arteritis (GCA), and polymyalgia rheumatica (PMR) are associated diseases. The major possible cause of FUO that is categorized as ‘miscellaneous’ is drug-associated fever [15].
According to a summary of clinical studies on FUO, the classification of diseases causing FUO differs between western countries and others. In western counties, the ratios of classifications causing FUO were 19, 24, 12, 8, and 38% for infections, NIID, malignancy, miscellaneous, and unknown, respectively; whereas in other countries, these have been reported as 43, 20, 14, 7, and 16%, respectively. The spectrum of diseases causing FUO has changed due to the widespread use of antibiotics and to new and improved diagnostic techniques such as CT, AUS, and echocardiography that contribute to early detection of the source. The major difference in causes of FUO between western counties and others is the proportion of infectious diseases, primarily tuberculosis. In western countries, the underlying cause of FUO in undiagnosed cases remains difficult to determine, because the use of advanced diagnostic techniques has enabled early diagnosis of FUO and thus earlier consultation in the majority of cases [3].
In children, infections are the major cause of FUO, accounting for 51% of cases according to a meta-analysis based on 18 studies [16, 17]. The trend in infections differs between developed countries, where bacterial infections, EBV, and Bartonella are representative; and that in developing countries, in which brucellosis, typhoid fever, tuberculosis, rickettsia infections, and abscesses are representative. Although malignancy occurs less frequently in children than in adults, lymphoma and leukemia are major causes of FUO in children, as is the case in adults. Autoimmune/rheumatologic conditions are also less frequent in children than in adults, and IBD, Crohn’s disease, and the systemic form of juvenile idiopathic arthritis are particular diseases that may cause FUO in children. Approximately 25–30% of children with FUO remain undiagnosed through the diagnostic workup for FUO, which is the same trend as in adults [16,17,18].
Figure 1 shows the transition of disease types causing FUO in adult patients in Japan [19,20,21,22]. Infectious disease was the major cause of FUO prior to 2004, but has decreased with time, along with gradual increases in NIID. The ratio of FUO caused by malignancy has not changed in recent 10 years. Undiagnosed cases have shown variation, but it has occurred at a large scale and constant rate. According to the latest survey in Japan (FDG-PET utilization rate, 31.2%), the major causes of FUO are lymphoma (8%), AOSD (5%), PMR (4%), vasculitis (4%), viral infection (4%), pericarditis (3%), and RA (3%). The number of cases of malignancy has increased in patients aged ≥ 65 years, half of which were malignant lymphoma [22].
Transition of disease types causing FUO in adult patients in Japan. Infectious disease is the major cause of FUO, but has decreased with time. Noninfectious inflammatory disease (NIID) has increased gradually as the cause of FUO. The ratio of FUO caused by malignancy has not changed in recent 10 years. Approximately 20% of FUO has ended with undiagnosed
Prognosis of patients with FUO
As FUO is caused by a variety of diseases, the overall prognosis depends on the background disease [23]. The mortality of immunocompetent patients with FUO in Belgium was 21–33% prior to 1980, decreasing to 6.5–16% during 1980–2000 and then to approximately 7% in the 2000s [24]. In Japan, the corresponding mortality rate was reported as 7% (9/121) in 2016–2017 [22]. Among the various causes of FUO, malignancy has the highest association with mortality. In the 2000s, the malignancy-associated mortality rate was 38–40%, which is much lower than that prior to 1980 (52–89%). Improvements in screening methods and the performance of diagnostic tools for malignancy might have been instrumental in the gradual decrease in mortality over time [24]. Lymphoma presenting as FUO tends to have a rapid progression and poor prognosis, and is difficult to diagnose [25]. Differences in prognosis among other diseases that cause FUO, with and without prolonged fever, are unknown. The prognosis of patients with FUO caused by NIID is a remaining issue. Considering the reduction in mortality during a half-century, improvements in screening methods and the establishment of clinical guidelines have enabled diagnosis of diseases with high mortality ratios. Cases without a final diagnosis may be caused by unknown diseases or a focus of fever that is undetectable even using current medical technology.
Tan studied the prognosis of 58 patients in 2004–2010, and reported that in 35 of 47 cases (74.5%) of undiagnosed FUO, the fever subsided during hospitalization or after discharge. Two patients required continued treatment with anti-inflammatory drugs and 10 patients (21.3%) died during follow-up, with 9 deaths caused by severe and worsening conditions related to the febrile illness [26]. Mulders-Manders et al. reported the prognosis of 131 cases of undiagnosed FUO among 274 patients with FUO, and found remission of fever in 47.3%. In this cohort, 66.7% of patients showed improvement by empirical treatment. Mortality occurred in 6.9% of the cohort, but appeared to have no relation with the febrile disease in most cases [27].
In FUO patients for whom a final diagnosis could not be obtained regardless of extensive investigation, prognosis was generally good and mortality was low [26, 27]. The same trend was confirmed in another study that found that patients with unexplained fever did not have serious illness [28]. In addition, 43–75% of adult patients with undiagnosed FUO experience spontaneous remission of fever [29,30,31,32], and empirical treatment with NSAIDs or corticosteroids increases this proportion [23].
Empirical administration of antimicrobial or anti-inflammatory therapy has been attempted blindly in patients with prolonged fever. For patients with unexplained FUO, antibiotics, NSAIDs, corticosteroids, and anakinra are major empirical treatments, and have been reported as effective in 60–66.7% of patients [26, 27]. However, therapeutic antimicrobial trials may cause a predisposition to resistance or suppress the growth of fastidious pathogens, and such treatment can be misleading as to the underlying cause of fever. Therefore, every possible attempt should be made to establish the diagnosis unless there is a rapid deterioration in clinical status [33].
Principles of FDG-PET/CT
FDG is a glucose-like substance in which the hydroxyl group at the 2-position of glucose is replaced with 18F, which is a positron-emitting nuclide. Like glucose, FDG is taken up into cells via glucose transporters and is phosphorylated by hexokinase, but, unlike glucose, is not metabolized by glycolysis and accumulates in the cell [34, 35]. In inflammatory cells, the glucose transporters GLUT1 and GLUT3 in the cell membrane are increased in the same manner as in malignant cells, and it is thought that glucose utilization is enhanced accordingly. In inflammation and infected foci, glucose consumption by activated inflammatory cells is 10 times greater than that in the inactivated state, which is the mechanism by which inflammatory tissue is visualized as high FDG accumulation [36,37,38].
Based on this biological background, FDG-PET and PET/CT are approved for use in Japan and are covered by national health insurance for staging of malignancy (except for early stage gastric cancer), screening for recurrence and metastasis of malignancy, and for diagnosis of the active lesion in cardiac sarcoidosis and large vessel aortitis (Takayasu arteritis and GCA).
As FDG shows high accumulation in both inflammatory diseases and malignant tumors, FDG-PET/CT examination enables identification of a causative focus of prolonged fever that is undetectable by other diagnostic imaging modalities. Even in situations where it is difficult to accurately identify a specific disease, FDG-positive areas have high probability of containing cancer and inflammatory cells, and thus, the pathological diagnosis is confirmed by accurate biopsy or puncture and the pathological diagnosis can be confirmed at an early stage, leading to early treatment of the FUO. Based on this mechanism, numerous clinical studies have demonstrated the utility of FDG-PET or FDG-PET/CT for the diagnosis of FUO.
Diagnostic workup for patients with FUO
The crucial process in the diagnostic workup in patients with FUO is to search for potential diagnostic clues (PDC), such as localizing signs, symptoms, and abnormalities pointing toward a diagnosis, through complete and repeated history-taking, and physical examination and obligatory investigations [3]. In addition, considering discontinuation or replacing medication to exclude drug fever is an important process.
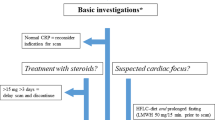
Figure 2 shows the diagnostic flow for FUO, which is modified from what is presented in Harrison’s principles of internal medicine [3]. Laboratory tests and imaging examinations are mainstream in the diagnostic process of FUO. Despite the high false-positive rate of US and low sensitivity of chest X-ray, these low-cost examinations remain obligatory in the diagnostic workup to separate patients in whom the cause of FUO is easily diagnosed. These are standard and widely performed examinations that can generally be completed in an outpatient visit. In the suggested flow of the diagnostic process of FUO, CT examination is regarded as later stage diagnostic test, along with bone marrow, temporal artery, and liver biopsies. The diagnostic yield of screening chest and abdominal CT in patients with FUO is low (< 20%), but the specificity is high. Although CT has limited additional value after a normal FDG-PET/CT, CT is a minimally invasive examination that may contribute to the diagnosis due to its high sensitivity [3]. However, according to clinical studies shown in later section, chest and abdominal CT examinations are frequently performed prior to scintigraphy or FDG-PET/CT. Considering the penetration rate and the cost, CT examinations have more accessibility than scintigraphy and FDG-PET/CT in the current clinical situation.
Diagnostic flow for FUO modified from Harrison’s principles of internal medicine. ESR erythrocyte sedimentation rate, CRP C-reactive protein, Hb hemoglobin, AST alanine aminotransferase, ALT aspartate aminotransferase, LDH lactate dehydrogenase, AUS abdominal ultrasonography, IGRA r interferon γ release assay, PDC potential diagnostic clues, NSAID Non-Steroidal Anti-Inflammatory Drugs
Performance of scintigraphy for the diagnosis of FUO
The basic concept of nuclear imaging is to noninvasively detect areas of functional changes in the total body. Representative nuclear imaging for the diagnosis of FUO has been 67Ga-citrate scintigraphy and 111In or 99mTc labeled leukocyte scintigraphy (LS) [3]. The sensitivity, specificity, and diagnostic yield of 67Ga-citrate scintigraphy was summarized based on 6 studies with 397 cases as 60% (95% CI 0.45–0.73), 63% (95% CI 0.37–0.84) and 35% (95% CI 0.25–0.46), respectively [39]. A direct comparison between FDG-PET/CT (sensitivity: 79%, specificity: 56%, accuracy: 72%, positive predictive value [PPV]: 83%, and negative predictive value [NPV]: 36%) and Ga single photon emission computed tomography (SPECT) /CT (sensitivity: 45%, specificity: 81%, accuracy: 55%, PPV: 86%, and NPV: 50%) revealed that FDG-PET/CT showed higher sensitivity and accuracy, and lower specificity, than Ga SPECT/CT. The positive contribution of FDG-PET/CT (72%) was higher than that of Ga SPECT/CT (55%). A high false-positive rate of 44% was observed for FDG-PET/CT, whereas a high false-negative rate of 55% was observed for Ga SPECT/CT [40]. Another prospective multicenter study showed the same trend (FDG-PET/CT: sensitivity: 45%, specificity: 40%, accuracy: 44%, PPV: 67%, and contribution: 33%; Ga SPECT/CT: sensitivity: 25%, specificity: 72%, accuracy: 38%, PPV: 71%, and contribution: 19%) [41]. Ga SPECT requires imaging at 48 to 72 h after 67Ga-citrate injection, which is longer than the time required for FDG-PET/CT (delay of 1–1.5 h after intravenous FDG injection followed by approximately 30 min of scanning). The estimated radiation exposure is higher for Ga SPECT (effective dose: 0.019 mSv/MBq) than for FDG-PET (effective dose: 0.1 mSv/MBq) [42].
The sensitivity, specificity, and diagnostic yield of LS for evaluating FUO was summarized based on 6 studies with 153 cases as 33% (95% CI 0.24–0.44), 83% (95% CI 0.61–0.94), and 20% (95% CI 0.14–0.28), respectively [39]. According to a direct comparison between FDG-PET and LS, FDG-PET has a higher sensitivity than LS in identifying the etiology of FUO (FDG-PET: sensitivity: 86%, specificity: 78%, PPV: 86%, and NPV: 78%, LS: sensitivity: 20%, specificity: 100%, PPV: 100%, and NPV: 40%) [43]. In another study, FDG-PET/CT showed higher sensitivity and substantial specificity with LS (FDG-PET/CT: sensitivity: 89.7%, specificity: 73.3%; and LS: sensitivity: 66.7%, specificity: 76.9%). The odds of positive findings increased 4.6-fold for PET/CT versus LS for helping the diagnosis of FUO [44]. The limitations of LS were (1) in vitro leukocyte labeling process requires skilled personnel and is not always available, (2) the risk of radiolabeling of leukocytes with the direct manipulation of blood products, (3) patients must visit the department on two consecutive days, and the count statistic detected with LS is weak in the images at 24 h after injection, and (4) complementary bone marrow imaging is usually required to improve accuracy [45,46,47]. In addition, the cost of a single dose of labeled leukocytes could be as much as 8–10 times that of a single dose of FDG [44]. Radiation exposure is estimated to be approximately 14 mSv in FDG-PET/CT, which is almost the same as for labeled leukocyte SPECT/CT, approximately 13 mSv (labeled leukocyte scan alone with approximately 7–8 mSv) [47,48,49]. Overall, FDG-PET/CT can assume the position occupied by Ga SPECT and LS in the diagnostic process of FUO.
Performance of FDG-PET/CT for the diagnosis of FUO
Numerous clinical studies and meta-analyses have presented the potential of FDG-PET/CT for the diagnosis of FUO. To understand the performance of FDG-PET/CT for the diagnosis of FUO, sensitivity and diagnostic yield (or contribution, as the proportion of patients in whom the imaging results were reported to contribute to the diagnosis of FUO causes) are definitely valid indexes among the various indexes applied in clinical studies. PPV is used to assess the incidence of false-positive (FP) findings in FDG-PET/CT, which can be caused by nonspecific or physiological FDG uptake. True negative (TN) cases are used to calculate specificity, NPV, and accuracy. Generally, TN is an outcome where the model correctly predicts the negative class. It is assumed that FDG-PET/CT is applied to cases of unknown cause of prolonged fever despite basic diagnostic workup. In FUO, not only does a specific reference standard not exist, but a final diagnosis might not be possible without the findings of FDG-PET/CT. Therefore, TN in FUO means a case with no abnormal findings on FDG-PET/CT in a case in which a final diagnosis was not attained through the diagnostic process, regardless that the cause of the prolonged fever was unsolved. Overall, TN is not thought of as a positive meaning, but as is presented in the previous section, true-negative cases may indicate a potentially good prognosis in patients with FUO. Therefore, the actual impacts of each of specificity, NPV, and accuracy should be reconsidered in the context of the diagnosis of FUO by FDG-PET/CT.
Ghady et al. reported that sensitivity and specificity of FDG-PET/CT for diagnosis of FUO based on four study results were 86–98% and 52–85%, respectively [33]. Kan et al. conducted a meta-analysis (based on 23 papers, 1927 cases) of FDG-PET/CT for diagnosis of FUO and reported sensitivity of 84% (95% CI 0.79–0.89), specificity of 63% (95% CI 0.49–0.75), a positive likelihood ratio of 2.3 (95% CI 1.5–3.4), and a negative likelihood of 0.25 (95% CI 0.16–0.38) [50]. Takeuchi et al. presented a meta-analysis (based on 42 papers, 2058 cases) of FDG-PET/CT for diagnosis of FUO, and reported sensitivity of 86% (95% CI 0.81–0.90), specificity of 52% (95% CI 0.36–0.37), and diagnostic yield of 58% (95% CI 0.51–0.64) [39]. Hao presented a meta-analysis (based on 15 papers, 595 cases) of FDG-PET/CT for diagnosis of FUO and reported sensitivity of 85% (95% CI 0.81–0.88) [51]. Dong et al. presented a meta-analysis (based on 9 papers, 388 cases) of FDG-PET or FDG-PET/CT for diagnosis of FUO, in which FDG-PET/CT showed sensitivity of 98.2% (95% CI 0.936–0.998), specificity of 85.9% (95% CI 0.750–0.934), positive likelihood ratio (LR) of 5.782 (95% CI 3.335–10.027), negative LR of 0.052 (95% CI 0.011–0.254), and diagnostic odds ratio (positive LR/negative LR) of 7.070 (95% CI 0.742–67.369) [52]. Bahrucha et al. found that the diagnostic yield of FDG-PET/CT was 56% (95% CI 0.50–0.61) (based on 18 papers, 905 cases) [53]. The results of these meta-analyses are in agreement that FDG-PET/CT has high sensitivity and moderate specificity for the diagnosis of FUO. Over half of patients with FUO are guided to a final diagnosis by FDG-PET/CT.
Abnormal findings on FDG-PET imaging increase the final diagnosis rate to 83%, which is higher than that of no particular findings on PET imaging, which has a rate of 36% (OR = 8.94 [95% CI 4.18–19.12]). Diagnostic performance for FUO was not influenced by PET or PET/CT, region, or follow-up period, but there was a difference between prospective (OR = 2.92 [95% CI 1.00–8.53]) and retrospective studies (OR = 18.57 [95% CI 7.57–45.59]) [54]. The higher proportion of neoplasms and infections than other causes of FUO led to a higher diagnostic yield [39].
According to another meta-analysis, FDG-PET/CT could contribute to the diagnosis of various diseases that cause FUO. The diagnostic yield of FDG-PET/CT for infectious diseases was 77.2%, among which tuberculosis (15.4%), pneumonia (9.5%), bone and joint infection (5.4%), and intra-abdominal abscess (5.0%) were the major components. The diagnostic yield of FDG-PET/CT for NIID was 64.9%, among which vasculitis (22.8%), sarcoidosis (7.0%), AOSD (5.8%), and thyroiditis (4.7%) were the major components. The diagnostic yield of FDG-PET/CT for malignancy was 95.5%, and lymphoma (61.6%) was a major component [53].
In addition to evaluation of unknown sites of inflammation, clinical indications for FDG-PET/CT in FUO patients include (1) for evaluation of unknown sites of neoplastic disease as causes of systemic symptoms, (2) to guide biopsy, (3) for assessment of therapeutic efficacy, and (4) for assessment of prognostic value [55, 56]. However, more robust evidence will be required to recommend their utility.
FDG-PET/CT has an indirect impact on the diagnosis of FUO by excluding possible causes of FUO and narrowing down the range of diseases. In a study evaluating the clinical impact of FDG-PET/CT on the clinical practice for FUO, 66% of the FUO cases performed FDG-PET/CT were regarded as leading to a change in the treatment plan and 25% of them were regarded as leading to improvement of the certainty factor with respect to the final cases, even though FDG-PET resulted in moderate sensitivity and specificity for the diagnosis of FUO [37].
Regarding FUO in children, a review based on 2 studies with 76 cases found that pediatric patients with abnormal PET findings were approximately 17 times more likely to achieve a definite diagnosis than those with normal PET findings (OR: 16.59, 95% CI 6.7–41) [57]. Based on a study that included 110 children with FUO, Pijl et al. reported that FDG-PET/CT showed sensitivity of 85.5% (95% CI 0.74–0.93), specificity of 79.2% (95% CI 0.65–0.90), PPV of 84.1% (95% CI 0.75–0.90), and NPV of 80.9% (95% CI 0.69–0.89) for the diagnosis of FUO. Treatment modification was made after FDG-PET/CT in 53% of the cohort, but the ratio of mortality within 3 months after FDG-PET/CT was 7%. FDG-PET/CT revealed that the cause of FUO in children was most frequently infection (57.3%) such as endocarditis, followed by NIID (37.2%) such as systemic juvenile idiopathic arthritis, miscellaneous diseases (17.9%) such as inflammatory bowel disorder as a representative disease, and neoplasm (8.4%) [58]. Accordingly, FDG-PET/CT can contribute to diagnosis in pediatric as well as adult patients with FUO.
Results of 26 clinical FDG-PET/CT studies for the diagnosis of FUO
To evaluate the backgrounds and results in clinical studies of FDG-PET/CT for diagnosis of FUO, 26 clinical studies (Table 1) [59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84] were selected that satisfied the following conditions: (1) targeting FUO (not mixed with inflammation of unknown origin [IUO]), (2) used FDG-PET/CT for the diagnosis of FUO, (3) provided the FDG-PET/CT results for cases that were ultimately undiagnosed, and (4) sample size > 10). The performance of FDG-PET/CT in selected 26 articles were listed in Table 2. The total number of enrolled case was 1637, and the mean number of enrolled cases per study was 63 ± 57 (range 12–303) in the selected clinical studies. The majority of the clinical studies were retrospective cohorts from a single institution. Major mandatory tests in the process of diagnosing FUO were blood test, blood culture, urine test, urine culture, and blood test regarding NIID. The mandatory imaging examinations were chest X-ray, AUS, and chest and abdominal CT. Therefore, FUO patients who underwent FDG-PET/CT had remained undiagnosed throughout these diagnostic processes. The etiology of disease classification causing FUO in these studies was highest in infection (33%), following NIID (23%), malignancy (14%), and miscellaneous (3%). The ratio of FUO patients was remained undiagnosed after FDG-PET/CT was 27%. The median values of sensitivity, specificity, PPV, accuracy, and diagnostic yield were 84.5, 60, 83, 73, and 50%, respectively; calculated by the number of cases, these values were 83, 64, 81, 76, and 54%, respectively. Even in a small number of studies with a prospective cohort, specificity, PPV, and diagnostic yield were lower than in those with a retrospective cohort. The diagnostic yield was higher in studies without mandatory tests than with them, and was also higher in studies without mandatory imaging examinations than with them (Table 3). Therefore, performing laboratory and imaging tests prior to FDG-PET/CT might provide a diagnosis in some patients, and only those in whom the diagnosis is difficult would require FDG-PET/CT. Nevertheless, FDG-PET/CT could identify the source of fever in half of the remaining undiagnosed patients.
In these studies, the definitions of false negative (FN), FP, and TN for FDG-PET/CT were not unified. In the case that FDG-PET/CT showed pathological findings that did not relate to the cause of fever, the case was commonly regarded either as FP (identified an area not related to the cause of FUO) or FN (could not identify the cause of FUO). Therefore, for further analysis, true positive (TP), FN, FP, and TN are redefined as follows: TP: FDG-PET/CT findings confirmed pathologically as the cause of the fever; FN: cause of fever unidentifiable by FDG-PET/CT in the case of confirmed pathological diagnosis; FP: positive FDG-PET/CT findings in the case of undetermined pathological diagnosis; and TN: etiology of fever undetermined both pathologically and on FDG-PET/CT.
The sensitivity, specificity, PPV, accuracy, and diagnostic yield of FDG-PET/CT were 75, 71, 86, 74, and 53%, respectively, calculated by the number of cases (Tables 2, 3).
FDG-PET/CT protocol and possible factors related to diagnostic results
A basic FDG-PET/CT examination protocol for investigation of FUO is provided in the European Association of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging (EANM/SNMMI) guidelines [85]. Considering the attributes of FDG-PET/CT imaging for detection of background diseases causing FUO, whole-body imaging is performed from vertex to toe. A deficiency of clinical information in patients with FUO may suppress the diagnostic performance of FDG-PET/CT [41]. The administration of antibiotics appears to have no clinically significant impact on the diagnostic accuracy of FDG-PET/CT performed for evaluation of known or suspected infectious processes, whereas administration of corticosteroids may lead to false-negative results in the case of FUO caused by systemic disease. [86]. Tsuzuki et al. reported that the diagnostic yield of FDG-PET/CT in patients with FUO or IUO was not related to age, sex, symptom duration, maximum body temperature, CRP, WBC count, ESR, Hb, or sIL-2 [87]. Garcia-Vincente et al. found that FDG-PET/CT results (positive or negative) were associated with the final diagnosis and with positive culture results, and that positive inflammation markers and protein analysis alterations were potent factors but not significantly so. Regarding the diagnostic performance of FDG-PET/CT, three or more positive inflammation markers and pathologic protein analysis were potent factors but not statistically significant, and maximum body temperature and positive serology were not related to the performance of FDG-PET/CT [78]. Crouzet et al. found that CRP > 30 mg/L and anemia were significantly associated with a performance of PET. The success rate of FDG-PET/CT was significantly higher in patients with constitutional symptoms and with suspected malignancy, whereas age, sex, duration of fever, CRP, leukocytes, and previous antibiotic treatment were not related to the success rate of FDG-PET/CT [68].
Balink et al. reported that elevated CRP levels < 20 mg/L were more predictive of positive FDG-PET/CT than ESR levels < 20 mm/h, and that FDG-PET/CT was 100% true negative in patients with CRP levels < 5 mg/L [88]. In another report, the diagnostic value of FDG-PET/CT increased in the conditions of presence of fever on the day of the scan and of the presence of elevated CRP within 7 days before the scan [89].
Regarding children with FUO, CRP was positively but weakly associated with identification of a true positive focus of fever on FDG-PET/CT (OR = 1.01 (95% CI 1.00–1.02) per mg/L increase in CRP) [58]. Overall, no significant index appears to be related to the diagnostic yield of FDG-PET/CT.
Because qualitative assessment is performed for the identification of any pathological FDG uptake related to the cause of prolonged fever, sites of para-physiological radiopharmaceutical uptake may be missed on FDG-PET/CT [56]. False-negative FDG-PET or PET/CT results can occur in systemic lupus erythematosus, cytomegalovirus infection, toxoplasmosis, urinary infection, septicemia, pyelonephritis, and Crohn’s disease [90]. PMR and AOSD have been reported as the major causes for which FDG-PET/CT most often showed no pathologic uptake leading to diagnosis [39](Fig. 3).
Typical FDG-PET imaging feature of PMR and AOSD. A Polymyalgia rheumatica (PMR): FDG uptake is confirmed at ischial tuberosity, greater trochanters, interspinous bursae, hips, shoulders, and sternoclavicular joints. B Adult-onset Still’s disease (AOSD): intense FDG uptake is confirmed at bone marrow, lymph nodes, and spleen
According to the 2012 provisional classification criteria for polymyalgia rheumatica, several disease symptoms and laboratory test results (CRP, ESR, rheumatoid factor, and anti-cyclic citrullinated peptide antibody) and findings have been proposed in the US as the criteria for classification of PMR [88], but are not intended for diagnostic use [91]. Although fever can occur in patients with PMR, it is not included in the criteria as a symptom indicating PMR; rather, GCA should be suspected [90]. A pathological diagnosis is not a decisive factor in the diagnosis of PMR. FDG-PET/CT is useful for the diagnosis of PMR because of the specific findings of FDG uptake at the ischial tuberosity, greater trochanters, interspinous bursae, hips, shoulders, and sternoclavicular joints [92, 93]. Therefore, FDG uptake distribution patterns and morphology can support a definitive diagnosis of PMR, and it is also useful for differentiating between PMR and elderly onset rheumatoid arthritis. However, the site of FDG accumulation may not always correlate with the clinical symptoms, and there is a possibility of subclinical conditions and the spread of other inflammation.
“Fever of at least 39 °C for at least a week” is one of the major diagnostic criteria of AOSD [94]. The diagnosis of AOSD is often difficult and time-consuming because of the absence of specific clinical, radiological, biological, and pathological characteristics [95]. The typical FDG-PET/CT findings in AOSD are uptake in bone marrow, lymph nodes, and spleen, and the intensity of FDG uptake is correlated with disease activity [96]. The differential diagnosis for AOSD includes infectious disease, malignant disease, and systemic disease [97], and lymphoma and viral infections that can cause FUO show a similar pattern of FDG uptake as for AOSD. The pathological findings of AOSD in lymph nodes are lymphadenitis or lymphatic hyperplasia to varying degrees. Even though FDG-PET/CT can identify pathological lymph nodes and lead to biopsy, the low specificity of the pathological findings is less useful for the diagnosis of AOSD [98], even though it is significant to exclude the diagnoses of malignant lymphoma and infectious lymphadenitis, which should be differentiated in the diagnostic process of AOSD. Therefore, FDG-PET/CT has limitations in the definitive diagnosis of AOSD in terms of image interpretation.
Outcomes in patients with FUO after FDG-PET/CT
In patients with FUO who obtain a final diagnosis by FDG-PET/CT, the prognosis depends on the underlying disease. FDG-PET/CT has been reported to reduce mortality rates in patients with infection such as staphylococcus aureus bacteremia, Gram-positive bacteremia, and pacemaker or defibrillator infection [99,100,101]. Therefore, FDG-PET/CT may have been a gamechanger in leading to a better prognosis for patients with FUO, because it enables an earlier final diagnosis and earlier start of appropriate treatment than before. A certain percentage of FUO patients remains undiagnosed after FDG-PET/CT. Patients with negative FDG-PET/CT results were significantly more likely to present with spontaneous regression than those with positive results (summary RR = 5.6; 95% CI 3.4–9.2) [55], Therefore, observation can also be considered an option in the clinical process for undiagnosed FUO patients in the event that FDG-PET/CT shows no specific findings.
Cost-effectiveness
Nakayo et al. analyzed cost-effectiveness in the diagnosis of FUO and estimated the influence of FDG-PET/CT on this cost. The real cost of the FUO process before the PET/CT test was 11,167.35€ per patient, which included 9620.82€ for 28.15 days of hospitalization, 151.63€ for 0.85 days of outpatient consultation, and 1394.90€ for complementary tests through diagnostic phases I–III and other tests. In contrast, the total theoretical cost of the FUO process before the FDG-PET/CT test was 7433.50€ per patient, which includes 5639.20€ for 16.50 theoretical hospitalization days, 178.78€ for 1 day of outpatient consultation, and 1615.52€ for complementary tests through diagnostic phases I–III. When FDG-PET/CT is performed in phase II (end of the second diagnostic week) in the FUO process, the theoretical cost of the entire FUO process is estimated to be 5696.22€, consisting of 4443.01€ for 13 theoretical days of hospitalization, 178.78€ for 1 day of outpatient consultation, and 1074.43€ for complementary tests through two diagnostic phases including FDG-PET/CT (500.00€). As a result, 5471€ per patient was estimated to be saved per patient [70].
Chen et al. evaluated the cost-effectiveness of FDG-PET/CT for FUO and IUO patients. The mean medical costs of the FDG-PET/CT group (23,556.96¥ [≒3337.47€]) were significantly higher than those of the non-FDG-PET/CT group (9266.65¥ [≒1312.87€]). However, a higher rate of definite diagnosis was achieved in the FDG-PET/CT group (91.4%) than in the non-FDG-PET/CT group (86.5%). The mean hospitalization days and mean medical costs before diagnosis were significantly lower in patients who had undergone FDG-PET/CT within 7 days after hospital admission than in those at 8 days or more after admission [102]. In another study of patients with IUO, the diagnostic rate was higher (with FDG-PET/CT: 70%, without FDG-PET/CT 30%) and cost per patient was lower (with FDG-PET/CT: 5298€, without FDG-PET/CT: 126,143€) in those who received FDG-PET/CT than in those who did not [103]. Accordingly, FDG-PET/CT appears to be cost-effective for patients with FUO, and efficiency might be improved if it is performed in a relatively early phase of the diagnostic process for FUO.
Status of clinical application of FDG-PET/CT for patients with FUO
The “Guideline for 18F-FDG Use in Inflammation and Infection” published for use in Europe and the United States state the usefulness of diagnosing inflammatory diseases [84]. The core SmPC and package leaflet guidelines for fludeoxyglucose (18F) published by the European Medicines Agency (EMA) documents “Localisation of abnormal foci guiding the aetiologic diagnosis in case of fever of unknown origin” as an indication for FDG. In the United Kingdom, Germany, and France, FDG has been designated as a “localization diagnosis of abnormal lesions that guides pathological diagnosis in fever of unknown origin” [104]. In the US, following the huge effort of SNMMI in approaching the Centers for Medicare and Medicaid Services (CMS), the CMS retired the National Coverage Determination (NCD) for 18F-FDG-PET for infection and inflammation (including FUO), which was effective on 1 January 2021, and updated in August 2021. As a result, coverage determinations for PET for infection and inflammation will be made at the discretion of local Medicare Administrative Contractors (MACs) [105, 106]. In Japan, FDG-PET/CT is not covered by national health insurance, but a prospective survey in Japan reported that FDG-PET/CT examinations were used in self-financed medical care for about 31% of patients with fever of unknown origin [22], and it is considered that this application will continue to progress. In the past 10 years, the availability of FDG-PET/CT has greatly expanded due to supporting clinical evidence. The appropriate use of FDG-PET/CT for patients with FUO is therefore expected to increase in the future.
FDG-PET/CT applied to the diagnosis of IUO
The term IUO is defined as FUO with a temperature not exceeding 38.3 °C, accompanied by elevated inflammatory markers on several occasions. The diagnostic approaches used for IUO are identical to those recommended for FUO [107]. The review of Affronni et al. found that the diagnostic approach taken for patients with FUO can also be applied to patients with low-grade fever (body temperature of 37.5–38.3 °C) on the basis that there was no relationship between body temperature values and severity of the underlying disease, and the disease spectrum was identical to that of FUO [108]. Vanderschueren et al. reported that the diagnostic yield, case mix, contribution of FDG-PET, and vital outcomes were similar between IUO and FUO [109]. FDG-PET/CT has shown comparable diagnostic sensitivity, specificity, and accuracy for IUO as for FUO [110,111,112]. In addition, numerous clinical studies that analyzed the performance of FDG-PET/CT for the diagnosis of combined FUO and IUO patients have reported similar utility of FDG-PET/CT as that of FUO patients alone [113,114,115,116,117,118,119,120,121,122].
Conclusion
FDG-PET/CT can be applied to patients with an unknown cause of prolonged fever despite basic diagnostic workup. High sensitivity and relative specificity have been reported, and diagnostic yield is expected for over half of FUO patients. In patients who remained undiagnosed after FDG-PET/CT, those with negative FDG-PET/CT results tend to have frequent spontaneous regression of fever. The availability of FDG-PET/CT has greatly expanded in the past 10 years, supported by clinical evidence. Based on this knowledge, the appropriate use of FDG-PET/CT for future patients with FUO is expected.
References
Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore). 1961;40:1–30.
Durack DT, Street AC. Fever of unknown origin—reexamined and redefined. Curr Clin Top Infect Dis. 1991;11:35–51.
HARRISON’S principles of internal medicine (20Th edition) volume 1, pp 114–122
Mackowiak PA. Concepts of fever. Arch Intern Med. 1998;158:1870–81.
Mackowiak PA, Boulant JA. Fever’s glass ceiling. Clin Infect Dis. 1996;22:525–36.
Roth J, Blatteis CM. Mechanisms of fever production and lysis: lessons from experimental LPS fever. Compr Physiol. 2014;4:1563–604.
Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–49.
Wright WF, Auwaerter PG. Fever and fever of unknown origin: review, recent advances, and lingering dogma. Open Forum Infect Dis. 2020;7:ofaa132.
Finetti F, Travelli C, Ercoli J, Colombo G, Buoso E, Trabalzini L. Prostaglandin E2 and cancer: insight into tumor progression and immunity. Biology (Basel). 2020;9:434.
Haidar G, Singh N. Fever of unknown origin. N Engl J Med. 2022;386:463–77.
Zhou W, Tan X, Li Y, Tan W. Human herpes viruses are associated with classic fever of unknown origin (FUO) in Beijing patients. PLoS ONE. 2014;9: e101619.
Haidar G. HHV-6, HHV-7, and HHV-8: forgotten viruses in transplantation. In: Morris M, Kotton C, Wolfe C, editors. Emerging transplant infections. Cham: Springer; 2020. p. 683–708.
Loizidou A, Aoun M, Klastersky J. Fever of unknown origin in cancer patients. Crit Rev Oncol Hematol. 2016;101:125–30.
van Kempen TS, Wenink MH, Leijten EFA, Radstake TRDJ, Boes M. Perception of self: distinguishing autoimmunity from autoinflammation. Nat Rev Rheumatol. 2015;11:483–92.
Williams J, Bellamy R. Fever of unknown origin. Clin Med (Lond). 2008;8:526–30.
Chow A, Robinson JL. Fever of unknown origin in children: a systematic review. World J Pediatr. 2011;7:5–10.
Attard L, Tadolini M, De Rose DU, Cattalini M. Overview of fever of unknown origin in adult and paediatric patients. Clin Exp Rheumatol. 2018;36(Suppl 110):10–24.
Gaeta GB, Fusco FM, Nardiello S. Fever of unknown origin: a systematic review of the literature for 1995–2004. Nucl Med Commun. 2006;27:205–11.
Ohshima H, Naito T, Kukino J, Fukuda Y, Sakamoto N, Mitsuhashi K, et al. An analysis of the 215patients with fever of unknown origin. Juntendo Med J. 2005;51:167–73.
Yamanouchi M, Uehara Y, Yokokawa H, Hosoda T, Watanabe Y, Shiga T, et al. Analysis of 256 cases of classic fever of unknown origin. Intern Med. 2014;53:2471–5.
Naito T, Mizooka M, Mitsumoto F, Kanazawa K, Torikai K, Ohno S, et al. Diagnostic workup for fever of unknown origin: a multicenter collaborative retrospective study. BMJ Open. 2013;3: e003971.
Naito T, Tanei M, Ikeda N, Ishii T, Suzuki T, Morita H, et al. Key diagnostic characteristics of fever of unknown origin in Japanese patients: a prospective multicentre study. BMJ Open. 2019;9: e032059.
Mulders-Manders C, Simon A, Bleeker-Rovers C. Fever of unknown origin. Clin Med (Lond). 2015;15:280–4.
Vanderschueren S, Eyckmans T, De Munter P, Knockaert D. Mortality in patients presenting with fever of unknown origin. Acta Clin Belg. 2014;69:12–6.
Zhang J, Chen B, Xu X, Lin Z, Huang B, Song J, Lin G. Clinical features of 66 lymphoma patients presenting with a fever of unknown origin. Intern Med. 2012;51:2529–36.
Tan Y, Liu X, Shi X. Clinical features and outcomes of patients with fever of unknown origin: a retrospective study. BMC Infect Dis. 2019;27(19):198.
Mulders-Manders C, Engwerda C, Simon A, van der Meer JWM, Bleeker-Rovers CP. Long-term prognosis, treatment, and outcome of patients with fever of unknown origin in whom no diagnosis was made despite extensive investigation: a questionnaire based study. Medicine (Baltimore). 2018;97: e11241.
Statler VA, Marshall GS. Characteristics of patients referred to a pediatric infectious diseases clinic with unexplained fever. J Pediatric Infect Dis Soc. 2016;5:249–56.
Bleeker-Rovers CP, Vos FJ, de Kleijn EM, et al. A prospective multicenter study on fever of unknown origin: the yield of a structured diagnostic protocol. Medicine (Baltimore). 2007;86:26–38.
Knockaert DC, Vanderschueren S, Blockmans D. Fever of unknown origin in adults: 40 years on. J Intern Med. 2003;253:263–75.
Zenone T. Fever of unknown origin in adults: evaluation of 144 cases in a non-university hospital. Scand J Infect Dis. 2006;38:632–8.
de Kleijn EM, Vandenbroucke JP, van der Meer JW. Fever of unknown origin (FUO). I. A prospective multicenter study of 167 patients with FUO, using fixed epidemiologic entry criteria. The Netherlands FUO Study Group. Medicine (Baltimore). 1997;76:392–400.
Ghady H, Nina S. Fever of unknown origin. NEJM. 2022;386:463–77.
Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–93.
Minamimoto R, Theeraladanon C, Suzuki A, Inoue T. Positron emission tomography for future drug development. Recent Patents Med Imaging. 2011;1:137–51.
Mochizuki T, Tsukamoto E, Kuge Y, et al. FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med. 2001;42:1551–5.
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–80.
Gamelli RL, Liu H, He LK, Hofmann CA. Augmentations of glucose uptake and glucose transporter-1 in macrophages following thermal injury and sepsis in mice. J Leukoc Biol. 1996;59:639–47.
Takeuchi M, Issa J, Dahabreh IJ, Nihashi T, Iwata M, Varghese GM, Terasawa T. Nuclear imaging for classic fever of unknown origin: meta-analysis. J Nucl Med. 2016;57:1913–9.
Hung BT, Wang PW, Su YJ, Huang WC, Chang YH, Huang SH, Chang CC. The efficacy of 18F-FDG PET/CT and 67Ga SPECT/CT in diagnosing fever of unknown origin. Int J Infect Dis. 2017;62:10–7.
Kubota K, Tanaka N, Miyata Y, Ohtsu H, Nakahara T, Sakamoto S, et al. Comparison of 18F-FDG PET/CT and 67Ga-SPECT for the diagnosis of fever of unknown origin: a multicenter prospective study in Japan. Ann Nucl Med. 2021;35:31–46.
ICRP Publication 128. Radiation dose to patients from radiopharmaceuticals: a compendium of current information related to frequently used substances. 2015.
Seshadri N, Sonoda LI, Lever AM, Balan K. Superiority of 18F-FDG PET compared to 111In-labelled leucocyte scintigraphy in the evaluation of fever of unknown origin. J Infect. 2012;65:71–9.
Dibble EH, Yoo DC, Baird GL, Noto RB. FDG PET/CT of infection: should it replace labeled leukocyte scintigraphy of inpatients? AJR Am J Roentgenol. 2019;213:1358–65.
Callahan RJ, Chilton HM, Ponto JA, et al. Procedure guideline for the use of radiopharmaceuticals. J Nucl Med Technol. 2007;35:272–5.
Salmanoglu E, Kim S, Thakur ML. Currently available radiopharmaceuticals for imaging infection and the holy grail. Semin Nucl Med. 2018;48:86–99.
Burroni L, Evangelista L. Should FDG PET/CT or PET/MR replace WBC scan in infectious and inflammatory disease? Clin Transl Imaging. 2021;9:551–2.
Censullo A, Vijayan T. Using nuclear medicine imaging wisely in diagnosing infectious diseases. Open Forum Infect Dis. 2017;4:1–7.
Larkin AM, Serulle Y, Wagner S, et al. Quantifying the increase in radiation exposure associated with SPECT/CT compared to SPECT alone for routine nuclear medicine examinations. Int J Mol Imaging 2011:897202.
Kan Y, Wang W, Liu J, Yang J, Wang Z. Contribution of 18F-FDG PET/CT in a case-mix of fever of unknown origin and inflammation of unknown origin: a meta-analysis. Acta Radiol. 2019;60:716–25.
Hao R, Yuan L, Kan Y, Li C, Yang J. Diagnostic performance of 18F-FDG PET/CT in patients with fever of unknown origin: a meta-analysis. Nucl Med Commun. 2013;34:682–8.
Donga M, Zhaoa K, Liua Z, Wanga G, Yanga S, Zhoub G. A meta-analysis of the value of fluorodeoxyglucose-PET/PET-CT in the evaluation of fever of unknown origin. Eur J Radiol. 2011;80:834–44.
Bharucha T, Rutherford A, Skeoch S, Alavi A, Brown M, Galloway J. The FDG-PET/CT in fever of unknown origin working group. Diagnostic yield of FDG-PET/CT in fever of unknown origin: a systematic review, meta-analysis, and Delphi exercise. Clin Radiol. 2017;72:764–71.
Besson FL, Chaumet-Riffaud P, Playe M, Noel N, Lambotte O, Goujard C, et al. Contribution of 18F-FDG PET in the diagnostic assessment of fever of unknown origin (FUO): a stratification-based meta-analysis. Eur J Nucl Med Mol Imaging. 2016;43:1887–95.
Takeuchi M, Nihashi T, Gafter-Gvili A, García-Gómez FJ, Andres E, Blockmans D, et al. Association of 18F-FDG PET or PET/CT results with spontaneous remission in classic fever of unknown origin: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97: e12909.
Casali M, Lauri C, Altini C, Bertagna F, Cassarino G, Cistaro A, et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: a guide for image acquisition and interpretation. Clin Transl Imaging. 2021;9:299–339.
Li Q, Tian R, Wang H, Li L, Wu T, Ren Y, Su M, Zou K, Sun X. Quantifying the contribution of 18F-FDG PET to the diagnostic assessment of pediatric patients with fever of unknown origin: a systematic review and meta-analysis. Pediatr Radiol. 2022.
Pijl JP, Kwee TC, Legger GE, Peters HJH, Armbrust W, Schölvinck EH, Glaudemans AWJM. Role of FDG-PET/CT in children with fever of unknown origin. Eur J Nucl Med Mol Imaging. 2020;47:1596–604.
Keidar Z, Gurman-Balbir A, Gaitin D, Israel O. Fever of unknown origin: the role of 18F-FDG PET/CT. J Nucl Med. 2008;49:1980–5.
Balink H, Collins J, Bruyn GA, Gemmel F. F-18 FDG PET/CT in the diagnosis of fever of unknown origin. Clin Nucl Med. 2009;34:862–8.
Federici L, Blondet C, Imperiale A, Sibilia J, Pasquali J-L, Pflumio F, et al. Value of (18)F-FDG-PET/CT in patients with fever of unknown origin and unexplained prolonged inflammatory syndrome: a single centre analysis experience. Int J Clin Pract. 2010;64:55–60.
Ferda J, Ferdová E, Záhlava J, Matejovic M, Kreuzberg B. Fever of unknown origin: a value of 18F-FDG-PET/CT with integrated full diagnostic isotropic CT imaging. Eur J Radiol. 2010;73:518–25.
Kei PL, Kok TY, Padhy AK, Ng DC, Goh AS. [18F] FDG PET/CT in patients with fever of unknown origin: a local experience. Nucl Med Commun. 2010;31:788–92.
Sheng JF, Sheng ZK, Shen XM, Bi S, Li JJ, Sheng GP, et al. Diagnostic value of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in patients with fever of unknown origin. Eur J Intern Med. 2011;22:112–6.
Pelosi E, Skanjeti A, Penna D, Arena V. Role of integrated PET/CT with [18F]-FDG in the management of patients with fever of unknown origin: a single-centre experience. Radiol Med. 2011;116:809–20.
Ergül N, Halac M, Cermik TF, Ozaras R, Sager S, Onsel C, et al. The diagnostic role of FDG PET/CT in patients with fever of unknown origin. Mol Imaging Radionucl Ther. 2011;20:19–25.
Kim YJ, Kim SI, Hong K-W, Kang MW. Diagnostic value of 18F-FDG PET/CT in patients with fever of unknown origin. Intern Med J. 2012;42:834–7.
Crouzet J, Boudousq V, Lechiche C, Pouget JP, Kotzki PO, Collombier L, et al. Place of (18)F-FDG-PET with computed tomography in the diagnostic algorithm of patients with fever of unknown origin. Eur J Clin Microbiol Infect Dis. 2012;31:1727–33.
Pedersen TI, Roed C, Knudsen LS, Loft A, Skinhoj P, Nielsen SD. Fever of unknown origin: a retrospective study of 52 cases with evaluation of the diagnostic utility of FDG-PET/CT. Scand J Infect Dis. 2012;44:18–23.
Nakayo EMB, Vicente AMG, Castrejón AMS, Narváez JAM, Rubio MPT, García VMP, et al. Analysis of cost-effectiveness in the diagnosis of fever of unknown origin and the role of (18)F-FDG PET-CT: a proposal of diagnostic algorithm. Rev Esp Med Nucl Imagen Mol. 2012;31:178–86.
Manohar K, Mittal BR, Jain S, Sharma A, Kalra N, Bhattacharya A, et al. F-18 FDG-PET/CT in evaluation of patients with fever of unknown origin. Jpn J Radiol. 2013;31:320–7.
Tokmak H, Ergonul O, Demirkol O, Cetiner M, Ferhanoglu B. Diagnostic contribution of (18)F-FDG-PET/CT in fever of unknown origin. Int J Infect Dis. 2014;19:53–8.
Gafter-Gvili A, Raibman S, Grossman A, Avni T, Paul M, Leibovici L, et al. [18F]FDG-PET/CT for the diagnosis of patients with fever of unknown origin. QJM. 2015;108:289–98.
Buch-Olsen KM, Andersen RV, Hess S, Braad PE, Schifter S. 18F-FDG-PET/CT in fever of unknown origin: clinical value. Nucl Med Commun. 2014;35(9):955–60.
Singh N, Kumar R, Malhotra A, Bhalla AS, Kumar U, Rita SR. Diagnostic utility of fluorodeoxyglucose positron emission tomography/computed tomography in pyrexia of unknown origin. Indian J Nucl Med. 2015;30:204–12.
Pereira AMV, Husmann L, Sah BR, Battegay E, Franzen D. Determinants of diagnostic performance of 18F-FDG PET/CT in patients with fever of unknown origin. Nucl Med Commun. 2016;37:57–65.
Hung BT, Wang PW, Su YJ, Huang WC, Chang YH, Huang SH, et al. The efficacy of 18F-FDG PET/CT and 67Ga SPECT/CT in diagnosing fever of unknown origin. Int J Infect Dis. 2017;62:10–7.
García-Vicente AM, Tello-Galán MJ, Amo-Salas M, Ros-Izquierdo J, German Andrés Jiménez-Londoño GA, Salas BLR, et al. Do clinical and laboratory variables have any impact on the diagnostic performance of 18F-FDG PET/CT in patients with fever of unknown origin? Ann Nucl Med. 2018;32:123–31.
Okuyucu K, Alagoz E, Demirbas S, Ince S, Karakas A, Karacalioglu O, et al. Evaluation of predictor variables of diagnostic [18F] FDG-PET/CT in fever of unknown origin. Q J Nucl Med Mol Imaging. 2018;62:313–20.
Georga S, Exadaktylou P, Petrou I, Katsampoukas D, Mpalaris V, Moralidis EI, et al. Diagnostic value of 18F-FDG-PET/CT in patients with FUO. J Clin Med. 2020;9:2112.
Das S, Sathyendra S, Hephzibah J, Karuppusami R, Gunasekaran K, Shanthly N, et al. Utility of positron emission tomography-computed tomography in the evaluation of fever of unknown origin in a resource-limited tropical nation. World J Nucl Med. 2021;20:237–46.
Letertre S, Fesler P, Zerkowski L, Picot MC, Ribstein J, Guilpain P, et al. Place of the <sup>18</sup>F-FDG-PET/CT in the Diagnostic Workup in patients with classical fever of unknown origin (FUO). J Clin Med. 2021;10:3831.
Buchrits S, Gafter-Gvili A, Eynath Y, Bernstine H, Guz D, Avni T. The yield of F<sup>18</sup> FDG PET-CT for the investigation of fever of unknown origin, compared with diagnostic CT. Eur J Intern Med. 2021;93:50–6.
Mahajna H, Vaknin K, Ben Shimol J, Watad A, Abu-Much A, Mahroum N, et al. The utility of 18FDG-PET/CT in diagnosing fever of unknown origin: the experience of a large tertiary medical center. Int J Environ Res Public Health. 2021;18:5360.
Francois J, John B, Arturo C, Paul EC, Dominique D, Kevin JD, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med. 2013;54:647–58.
Kagna O, Kurash M, Ghanem-Zoubi N, Keidar Z, Israel O. Does antibiotic treatment affect the diagnostic accuracy of 18F-FDG PET/CT studies in patients with suspected infectious processes? J Nucl Med. 2017;58:1827–30.
Tsuzuki S, Watanabe A, Iwata M, Toyama H, Terasawa T. Predictors of diagnostic contributions and spontaneous remission of symptoms associated with positron emission tomography with fluorine-18-fluorodeoxy glucose combined with computed tomography in classic fever or inflammation of unknown origin: a retrospective study. J Korean Med Sci. 2021;36: e150.
Balink H, Veeger NJ, Bennink RJ, et al. The predictive value of C-reactive protein and erythrocyte sedimentation rate for 18F-FDG PET/CT outcome in patients with fever and inflammation of unknown origin. Nucl Med Commun. 2015;36:604–9.
Kouijzer IJE, Mulders-Manders CM, Bleeker-Rovers CP, Oyen WJG. Fever of unknown origin: the value of FDG-PET/CT. Semin Nucl Med. 2018;48:100–7.
Sioka C, Assimakopoulos A, Fotopoulos A. The diagnostic role of (18)F fluorodeoxyglucose positron emission tomography in patients with fever of unknown origin. Eur J Clin Invest. 2015;45:601–8.
Dasgupta B, Cimmino MA, Maradit-Kremers H, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Ann Rheum Dis. 2012;71:484–92.
González-Gay MA, Matteson EL, Castañeda S. Polymyalgia rheumatica. Lancet. 2017;390:1700–12.
van der Geest KSM, Treglia G, Glaudemans AWJM, Brouwer E, Jamar F, Slart RHJA, et al. Diagnostic value of [18F]FDG-PET/CT in polymyalgia rheumatica: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:1876–89.
Takahashi H, Yamashita H, Kubota K, Miyata Y, Okasaki M, Morooka M, et al. Differences in fluorodeoxyglucose positron emission tomography/computed tomography findings between elderly onset rheumatoid arthritis and polymyalgia rheumatica. Mod Rheumatol. 2015;25:546–51.
Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still’s disease. Nat Rev Rheumatol. 2018;14:603–18.
Gerfaud-Valentin M, Sève P, Hot A, Broussolle C, Jamilloux Y. Pathophysiology, subtypes, and treatments of adult-onset Still’s disease: an update. Rev Med Interne. 2015;36:319–27.
Brisset J, Jamilloux Y, Dumonteil S, Lades G, Killian M, Gerfaud-Valentin M, et al. Characteristics and clinical value of 18F-FDG PET/CT in the management of adult-onset still’s disease: 35 cases. J Clin Med. 2021;10:2489.
Kim HA, Kwon JE, Yim H, Suh CH, Jung JY, Han JH. The pathologic findings of skin, lymph node, liver, and bone marrow in patients with adult-onset still disease: a comprehensive analysis of 40 cases. Medicine (Baltimore). 2015;94: e787.
Berrevoets MAH, Kouijzer IJE, Aarntzen EHJG, Janssen MJR, De Geus-Oei LF, Wertheim HFL, Kullberg BJ, Oever JT, Oyen WJG, Bleeker-Rovers CP. 18F-FDG PET/CT optimizes treatment in Staphylococcus aureus bacteremia and is associated with reduced mortality. J Nucl Med. 2017;58:1504–10.
Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med. 2011;52:1673–8.
Diemberger I, Bonfiglioli R, Martignani C, Graziosi M, Biffi M, Lorenzetti S, Ziacchi M, Nanni C, Fanti S, Boriani G. Contribution of PET imaging to mortality risk stratification in candidates to lead extraction for pacemaker or defibrillator infection: a prospective single center study. Eur J Nucl Med Mol Imaging. 2019;46:194–205.
Chen JC, Wang Q, Li Y, Zhao YY, Gao P, Qiu LH, et al. Current situation and cost-effectiveness of 18F-FDG PET/CT for the diagnosis of fever of unknown origin and inflammation of unknown origin: A single-center, large-sample study from China. Eur J Radiol. 2022;148: 110184.
Balink H, Tan SS, Veeger NJ, Holleman F, van Eck-Smit BL, Bennink RJ, Verberne HJ. 18F-FDG PET/CT in inflammation of unknown origin: a cost-effectiveness pilot-study. Eur J Nucl Med Mol Imaging. 2015;42:1408–13.
European Medicines Agency; Guideline on core SmPC and Package Leaflet for fludeoxyglucose (18F), 19 July 2012, Committee for Medicinal Products for Human Use (CHMP). [cited 2022 May 12]. Available from: https://www.ema.europa.eu/en/documents/other/core-summary-product-characteristics-smpc-fludeoxyglucose_en.pdf.
Wahl RL, Dilsizian V, Palestro CJ. At Last, 18F-FDG for Inflammation and Infection! J Nucl Med. 2021;62:1048–9.
SNMMI, NEWS AND MEDIA. https://www.snmmi.org/NewsPublications/NewsDetail.aspx?ItemNumber=37176.
Balink H, Verberne HJ, Bennink RJ, van Eck-Smit BL. A rationale for the use of F18-FDG PET/CT in fever and inflammation of unknown origin. Int J Mol Imaging. 2012;2012: 165080.
Affronti M, Mansueto P, Soresi M, et al. Low-grade fever: how to distinguish organic from non-organic forms. Int J Clin Pract. 2010;64:316–21.
Vanderschueren S, Del Biondo E, Ruttens D, Van Boxelaer I, Wauters E, Knockaert DD. Inflammation of unknown origin versus fever of unknown origin: two of a kind. Eur J Intern Med. 2009;20:415–8.
Balink H, Bennink RJ, Veeger NJ, van Eck-Smit BL, Verberne HJ. Diagnostic utility of (18)F-FDG PET/CT in inflammation of unknown origin. Clin Nucl Med. 2014;39:419–25.
Bilici Salman R, Gülbahar Ateş S, Satiş H, Tufan A, Akdemir ÜÖ, Yapar D, et al. Diagnostic role of 18F-fluorodeoxyglucose positron emission tomography for the evaluation of patients with inflammation of unknown origin. J Clin Rheumatol. 2021;27:219–25.
Holubar J, Broner J, Arnaud E, Hallé O, Mura T, Chambert B, Sotto A, Roubille C, Gaujoux-Viala C, Goulabchand R. Diagnostic performance of 18 F-FDG-PET/CT in inflammation of unknown origin: a clinical series of 317 patients. J Intern Med. 2022;291:856–63.
Öğüt TS, Erbasan F, Terzioğlu ME, Tazegul G, Yazısız V. The diagnostic value of fluoro-18 fluorodeoxyglucose (F-18 FDG) PET/CT in fever or inflammation of unknown origin: a retrospective study at a rheumatology clinic. Cureus. 2022;6(14): e24192.
Chen JC, Wang Q, Li Y, Zhao YY, Gao P, Qiu LH, et al. Current situation and cost-effectiveness of <sup>18</sup>F-FDG PET/CT for the diagnosis of fever of unknown origin and inflammation of unknown origin: a single-center, large-sample study from China. Eur J Radiol. 2022;148: 110184.
Weitzer F, Nazerani Hooshmand T, Pernthaler B, Sorantin E, Aigner RM. Diagnostic value of F-18 FDG PET/CT in fever or inflammation of unknown origin in a large single-center retrospective study. Sci Rep. 2022;12:1883.
Ly KH, Costedoat-Chalumeau N, Liozon E, Dumonteil S, Ducroix JP, Sailler L, et al. Diagnostic value of 18F-FDG PET/CT vs. chest–abdomen–pelvis CT scan in management of patients with fever of unknown origin, inflammation of unknown origin or episodic fever of unknown origin: a comparative multicentre prospective study. J Clin Med. 2022;11:386.
Casali M, Lauri C, Altini C, Bertagna F, Cassarino G, Cistaro A, et al. State of the art of <sup>18</sup>F-FDG PET/CT application in inflammation and infection: a guide for image acquisition and interpretation. Clin Transl Imaging. 2021;9:299–339.
Wang WX, Cheng ZT, Zhu JL, Xing MY, Zheng CF, Wang SJ, et al. Combined clinical parameters improve the diagnostic efficacy of 18F-FDG PET/CT in patients with fever of unknown origin (FUO) and inflammation of unknown origin (IUO): a prospective study in China. Int J Infect Dis. 2020;93:77–83.
Mulders-Manders CM, Kouijzer IJ, Janssen MJ, Oyen WJ, Simon A, Bleeker-Rovers CP. Optimal use of [18F]FDG-PET/CT in patients with fever or inflammation of unknown origin. Q J Nucl Med Mol Imaging. 2021;65:51–8.
Kan Y, Wang W, Liu J, Yang J, Wang Z. Contribution of 18F-FDG PET/CT in a case-mix of fever of unknown origin and inflammation of unknown origin: a meta- analysis. Acta Radiol. 2019;60:716–25.
Wang Q, Li YM, Li Y, Hua FC, Wang QS, Zhang XL, et al. 18F-FDGPET/CT in fever of unknown origin and inflammation of unknown origin: a Chinese multi-center study. Eur J Nucl Med Mol Imaging. 2019;46:159–65.
Schönau V, Vogel K, Englbrecht M, Wacker J, Schmidt D, Manger B, et al. The value of <sup>18</sup>F-FDG-PET/CT in identifying the cause of fever of unknown origin (FUO) and inflammation of unknown origin (IUO): data from a prospective study. Ann Rheum Dis. 2018;77:70–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Ethical statement
Reference no. [41] which was performed in studies involving human participants was in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This review does not contain any other studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Minamimoto, R. Optimal use of the FDG-PET/CT in the diagnostic process of fever of unknown origin (FUO): a comprehensive review. Jpn J Radiol 40, 1121–1137 (2022). https://doi.org/10.1007/s11604-022-01306-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01306-w