Abstract
Stereotactic body radiotherapy (SBRT) has excellent local control and low toxicity for spinal metastases and is widely performed for spinal oligometastases. However, its additional survival benefit to standard of care, including systemic therapy, is unknown because the results of large-scale randomized controlled trials regarding SBRT for oligometastases have not been reported. Consequently, the optimal patient population among those with spinal oligometastases and the optimal methodology for spine SBRT remain unclear. The present review article discusses two topics: evidence-based optimal patient selection and methodology. The following have been reported to be good prognostic factors: young age, good performance status, slow-growing disease with a long disease-free interval, minimal disease burden, and mild fluorodeoxyglucose accumulation in positron emission tomography. In addition, we proposed four measures as the optimal SBRT method for achieving excellent local control: (i) required target delineation; (ii) recommended dose fraction schedule (20 or 24 Gy in a single fraction for spinal oligometastases and 35 Gy in five fractions for lesions located near the spinal cord); (iii) optimizing dose distribution for the target; (iv) dose constraint options for the spinal cord.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1995, Hellman and Weichselbaum hypothesized that oligometastasis (≤ 5 extracranial metastases) is an intermediate state along the spectrum between local and systemic diseases [1]. According to this hypothesis, oligometastases are pathophysiologically similar to local disease and may benefit from local treatment. Some reports have suggested that it can be cured by performing local treatment with curative intent for distant metastasis (i.e., lung metastases from sarcoma, colorectal liver metastases, and extraregional lymph node metastases from cervical cancer) [2,3,4].
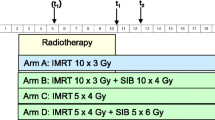
Stereotactic body radiotherapy (SBRT) with intensity-modulated radiotherapy and image-guidance techniques has emerged as a new treatment option for spinal metastases (Fig. 1) [5]. Spine SBRT achieves a high local control (LC) rate (1 year: 90%) and has low toxicity (0.2% rate of neurologic injury) [6]. For spinal oligometastases, high-precision radiotherapy is essential for curative dose administration because of the positional relationship between the spinal tumor and spinal cord. Several clinical guidelines have recommended SBRT for spinal oligometastases [6, 7], and the results of the SABR-COMET trial support these recommendations [8]. The SABR-COMET trial is the first randomized study to clarify the survival benefit of SBRT for oligometastases over standard of care (median survival time: 41 vs. 28 months, p = 0.09). However, that trial had some limitations: it was a phase 2 trial with insufficient sample size, while more patients with prostate cancer and less than 35% of spinal metastasis cases were included in the SBRT arm [8]. In addition, none of the multiple ongoing large-scale randomized controlled trials assessing SBRT for oligometastases have reported such results (Table 1) [9,10,11,12,13,14,15]. Therefore, the optimal patient population among those with spinal oligometastases and the optimal methodology of spine SBRT remain unclear. The present review article considers two topics: patient selection and optimal methodology based on evidence.
Patient selection
When performing SBRT with curative intent among patients with oligometastases, it is important to select patients with particularly good prognosis and curability potential. Inappropriate patient selection for spine SBRT poses unnecessary toxicity risks to patients, such as vertebral compression fracture (VCF), esophagitis, myelopathy, and radiculopathy.
Good prognosis
Several studies have reported on the prognostic factors after SBRT. Chao et al. generated a prognostic index based on the recursive partitioning analysis for patients treated with spine SBRT (not limited to oligometastases) [16]. Classified into three groups according to the time from primary diagnosis, Karnofsky performance status (PS), and age, the group with the best prognosis (time from primary diagnosis > 30 months and Karnofsky PS > 70) had a median survival time of 21.1 months. This result seems inadequate as a prognostic index.
Jensen et al. proposed the Prognostic Index for Spine Metastases, which was developed using data from prospective single institution trials on stereotactic spine radiosurgery [17]. The score accounts for sex, PS, previous therapy at the intended treatment site, number of organs involved, time elapsed between diagnosis and metastasis, and number of spine metastases. The scoring system categorizes patients into four groups, with a 5-year overall survival rate of 50–66% in the group with the best prognosis.
Zeng et al. compared patients who died within 3 months after spine SBRT and those who lived for > 3 years [18]. Shorter survival time after spine SBRT was observed in patients with non-breast or prostate primaries, Eastern Cooperative Oncology Group PS ≥ 2, polymetastatic disease, painful lesions, and paraspinal disease on multivariate analysis.
Although the prognostic factors of long-term survival after SBRT or surgery varied in previous studies, the identified prognostic factors tended to be related to four major overarching criteria: young age (< 65 or < 70 years), patient fitness (Karnofsky PS ≥ 70), slow-growing disease (i.e., a long disease-free interval prior to metastasis), and low disease burden (i.e., a smaller number of metastases or single-organ oligometastases) [19]. The director of the SABR-COMET trial termed these powerful prognostic factors for patients with oligometastatic disease as the “four aces” [19]. Indeed, the SABR-COMET included many patients with these characteristics (Eastern Cooperative Oncology Group PS 0–1; slow-growing disease, including prostate and breast cancer; long time from diagnosis of primary tumor to randomization; and one or two metastases), which may have been the reason for obtaining satisfactory outcomes [8].
A phase 2 trial that included 175 patients with oligometastases treated with SBRT analyzed good prognostic factors using diagnostic images [20]. They compared 5-year polymetastatic-free survival in three groups: (1) patients with a combination of < 14.8 mL oligometastatic tumor volume and a fluorodeoxyglucose (FDG) positron emission tomography (PET)-computed tomography (CT) maximum standardized uptake value (SUVmax) < 6.5; (2) patients with a combination of < 14.8 mL oligometastatic tumor volume and an FDG-PET/CT SUVmax ≥ 6.5; (3) patients with an oligometastatic tumor volume ≥ 14.8 mL regardless of the SUVmax. The 5-year polymetastatic-free survival rates were 89%, 58% (p = 0.02), and 17% (p < 0.001) in Groups 1, 2, and 3, respectively. These findings suggest that the total gross tumor volume (GTV) of the oligometastases and the SUVmax are useful in determining whether the patients have a potential risk of polymetastases.
Use of advanced diagnostic images
Advanced diagnostic imaging modalities are effective in accurately diagnosing a small number of metastases and excluding polymetastases. FDG-PET/CT and diffusion-weighted whole-body imaging with background signal suppression (DWIBS) magnetic resonance imaging are useful for diagnosing oligometastases because they are capable of whole-body evaluation and have the spatial resolution necessary to identify targets for SBRT [21]. In addition, these tests have higher sensitivity and specificity than bone scintigraphy [22]. Although PET-CT has the advantage of easily identifying lesions in the acquired images, its limitation lies in radionuclide accumulation in regions of high glucose metabolism, such as areas with non-specific inflammation and physiological changes other than tumors. For osteoblastic metastases, PET is less suitable than bone scintigraphy, and confirmation of osteosclerosis on CT is desirable [22]. DWIBS reflects the motion restriction of water molecules in areas of high cell density and is another tool for detecting metastasis [23]. The main advantage of DWIBS is that it is less invasive, as there is no exposure to radiation and images can be acquired without the use of contrast media [24]. However, lesions in bones with high signal intensity on T2-weighted images, such as hemangiomas or red bone marrow in patients with anemia, can be judged as abnormal signals. Such images require great skill for accurate interpretation [25, 26]. Previous reports assessing the use of PET-CT and DWIBS to detect bone metastases have shown that they have similar sensitivities; however, the specificity of DWIBS may be lower than that of PET-CT [27,28,29]. In addition, prostate-specific membrane antigen-PET is suitable for assessing bone metastasis in prostate cancer if the above tests do not provide satisfactory results [30].
Optimal methodology
The purposes of spine SBRT are as follows: complete control of oligometastasis, relief of painful lesions, and improvement of neurologic function for patients with epidural spinal cord compression [31]. Among these, SBRT for oligometastases must be an extremely aggressive treatment strategy and achieve a high LC rate because of its curative intent.
A previous report suggested the importance of SBRT methodologies for oligometastases. A phase 3 randomized trial comparing SBRT doses for oligometastases (more than 90% of patients had bone metastases) showed that high-dose SBRT (24 Gy) in a single fraction had a significantly lower local failure rate than medium-dose SBRT (27 Gy) in three fractions [32]. Notably, the cumulative incidence of distant metastatic progression was also significantly lower in the high-dose SBRT group (3 years: 5.3% vs. 22.5%, p = 0.01). Therefore, appropriate local treatment for the initial metastases may prevent progression to systemic disease. Herein, we suggested countermeasures to improve LC in patients with spinal oligometastatic disease receiving SBRT.
Delineation of the target
In the early days of spine SBRT, no clinical target volume (CTV) margin was popular [33,34,35]. However, a retrospective study showed that contouring the whole vertebral body tended to improve LC compared to contouring part of the vertebral body [36]. In addition, the International Spine Radiosurgery Consortium recommends CTV expansion based on the GTV location, wherein the spine is divided into six sectors (the vertebra, both pedicle, both transverse process, and spinous process) and the CTV includes the sectors where the GTV is located and the sectors adjacent to the GTV for subclinical tumor spread in the marrow space [37]. This standard setting should be enforced to achieve a high LC rate.
Dose fraction schedule
Various dose fractionations are used depending on the facility, and there is no broad consensus on the optimal dose fractionation in SBRT for spinal oligometastases [38]. A meta-analysis comparing the prescribed dose of spine SBRT showed that a higher cumulative dose led to a higher 2-year LC rate in patients receiving 1–5 fractions [39]. Dose fraction schedules should be selected from those used in the randomized controlled trials of the SABR-COMET trial and the dose comparison trial conducted by Zelefsky et al. (Table 2) [8, 32]. If the treatment goal is a 2-year LC rate > 80%, appropriate dose fractionations are 18 Gy/1 Fr, 20 Gy/1 Fr, 24 Gy/1 Fr, 30 Gy/3 Fr, and 35 Gy/5 Fr. The regimen with the highest LC rate is 24 Gy in a single fraction (estimated 2-year LC of 96%) [39].
In cases wherein the tumor is adjacent to the spinal cord [minimum distance between the GTV and planning organ-at-risk volume (PRV) of the cord < 3 mm [40]], we may have to increase the number of the fraction size of SBRT. A dose constraint of the spinal cord for the radiation-naive region is 12.4 Gy in a single fraction [41], resulting in a delivery of 27.78 Gy [biological equivalent dose with α/β = 10 (BED10)] to the epidural tumor in contact with the spinal cord. In a regimen involving five fractions, as the spinal cord dose constraint would be 25.3 Gy [41], 38.10 Gy (BED10) can be administered to the epidural tumors. By increasing fractionation, it is possible to escalate the minimum dose delivered to a gross tumor, which would contribute toward achieving LC [42,43,44]. However, the benefit of increasing fractionation may be limited or none in cancer types with low α/β such as breast and prostate cancers. It is noted that for high-grade epidural spinal cord compression, surgical intervention should precede SBRT to deliver a sufficiently high tumoricidal dose [45].
VCF is a relatively common adverse effect of spine SBRT. Several studies have identified that high dose per fraction is a risk factor for VCF development [38, 46]. A multi-institutional retrospective study showed that the cumulative incidence of VCF at 1 year was 39% with ≥ 24 Gy/fraction, 19% with 20–23 Gy/fraction, and 10% with ≤ 19 Gy/fraction [46]. Although increasing the number of fractions and reducing the dose per fraction are effective in avoiding VCF, it should be recognized that VCF is an adverse effect that is painless in most cases [47] and does not directly correlate with survival.
From the aforementioned studies, the recommended doses are 20 or 24 Gy in a single fraction for spinal oligometastases and 35 Gy in five fractions for lesions close to the spinal cord (minimum distance between GTV and PRV of the cord < 3 mm).
Optimizing dose distribution for the target
The prescribed dose is generally delivered up to 90–95% of the planning target volume (PTV) [8, 48]. In addition, the isodose prescription is used to sharply reduce the dose outside the PTV while administering a high dose within the PTV. Several dosimetric analyses have shown a positive correlation between LC and the marginal dose to the GTV but not the dose to the PTV [42,43,44]. Retrospective data from the MD Anderson Cancer Center showed that patients with a minimum dose to the GTV ≥ 14 Gy in a single fraction had a significantly higher LC rate than those with a minimum dose < 14 Gy [42]. In addition, retrospective data from the Memorial Sloan-Kettering Cancer Center suggested that the thresholds of local failure were a minimum dose to the GTV of < 15 Gy [43] and a dose that covered 95% of the GTV ≤ 18.3 Gy in a single fraction [44]. Therefore, the isodose prescription, which produces a steep dose gradient and a hotspot in the target, is valid concerning dose reduction to the organs at risk and excellent LC.
Optimizing the treatment plan increases the target dose coverage and reduces the inter- and intra-planner variability [41]. If the GTV is not located near the spinal cord, the GTV dose can be increased by setting a large limit on the maximum dose in the target compared to the prescribed dose (e.g., the protocol of CCTG SC.24/TROG 17.06 allows a maximum dose of + 50% in the PTV [47]). If the GTV is in contact with the spinal cord, the minimum GTV dose should be as close as possible to the spinal cord dose constraint (prioritize the dose constraint). The target dose coverage should be optimized based on past clinical trial protocols [48], with the goal of achieving at least 90–95% coverage of the PTV by the prescribed dose. The created dose distribution should be visually checked for a steep dose gradient around the spinal cord, considering that the maximum photon dose fall-off gradient is approximately 10–13% per millimeter [40].
Alleviation of the dose constraint for the spinal cord
Table 3 summarizes the representative dose constraints for the spinal cord in de novo SBRT [49,50,51,52,53,54]. There are three dose constraints of 12.4, 14, and 16 Gy in a single fraction at the maximum point dose (with point defined as ≤ 0.035 cc [50]). Several studies examining SBRT for de novo spinal metastases using the strictest constraint (maximum point dose of 17 Gy in two fractions for thecal sac or the PRV of the cord [49]) have not observed radiation myelopathy [55,56,57]. Reports that calculated dose constraint of 14 Gy have adopted the spinal cord itself (without PRV margin) as the structure of interest [50,51,52,53] (Table 3). Some reports that used this setting have not confirmed radiation myelopathy in the long-term follow-up examination [48, 58]. Alleviating the dose constraint of the spinal cord is an option for increasing the minimum dose delivered to an epidural tumor. However, radiation oncologists should use the 16 Gy dose constraint with caution in clinical practice because of the small sample size of this phase 1 trial [54].
Conclusion
Although SBRT can cure some patients with oligometastases, the optimal patient population and methodology for spine SBRT remain unknown. Patients with young age, good fitness, slow-growing disease, low disease burden, and mild FDG accumulation in PET are suitable for spine SBRT with curative intent. In addition, the use of appropriate diagnostic imaging can exclude the possibility of false oligometastases. Regarding the optimal methodology, we proposed four countermeasures to improve LC. We believe that this information will be useful when selecting patients and performing appropriate spine SBRT in daily clinical practice.
References
Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. https://doi.org/10.1200/JCO.1995.13.1.8.
Kim S, Ott HC, Wright CD, Wain JC, Morse C, Gaissert HA, et al. Pulmonary resection of metastatic sarcoma: prognostic factors associated with improved outcomes. Ann Thorac Surg. 2011;92:1780–6. https://doi.org/10.1016/j.athoracsur.2011.05.081 (discussion 1786).
Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. https://doi.org/10.1200/JCO.2007.11.0833.
Kim JY, Kim JY, Kim JH, Yoon MS, Kim J, Kim YS. Curative chemoradiotherapy in patients with stage IVB cervical cancer presenting with paraaortic and left supraclavicular lymph node metastases. Int J Radiat Oncol Biol Phys. 2012;84:741–7. https://doi.org/10.1016/j.ijrobp.2012.01.070.
Lutz S, Balboni T, Jones J, Lo S, Petit J, Rich SE, et al. Palliative radiation therapy for bone metastases: update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol. 2017;7:4–12. https://doi.org/10.1016/j.prro.2016.08.001.
Husain ZA, Sahgal A, De Salles AD, Funaro M, Glover J, Hayashi M, et al. Stereotactic body radiotherapy for de novo spinal metastases: systematic review. J Neurosurg Spine. 2017;27:295–302. https://doi.org/10.3171/2017.1.SPINE16684.
Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–76. https://doi.org/10.1016/j.ijrobp.2010.11.026.
Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-comet): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–8. https://doi.org/10.1016/S0140-6736(18)32487-5.
Standard of care therapy with or without stereotactic radiosurgery and/or surgery in treating patients with limited metastatic breast cancer. NCT02364557. Clini-calTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02364557. Accessed 22 Dec 2021
Maintenance chemotherapy with or without stereotactic body radiation therapy in treating patients with Stage IV non-small cell lung cancer. NCT03137771. Clini-calTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03137771. Accessed 22 Dec 2021
Stereotactic ablative radiotherapy for Oligometastatic non-small cell lung cancer (SARON). Clin. Trials.gov NCT02417662. https://clinicaltrials.gov/ct2/show/NCT02417662. Accessed 22 Dec 2021
Conventional care versus Radioablation (stereotactic body radiotherapy) for extracranial Oligometastases (CORE). Clin. Trials.gov NCT02759783. https://clinicaltrials.gov/ct2/show/NCT02759783. Accessed 22 Dec 2021
Stereotactic ablative radiotherapy for comprehensive treatment of Oligometastatic (1–3 metastases) cancer (SABR-COMET-3). Clin. Trials.gov NCT03862911. https://clinicaltrials.gov/ct2/show/NCT03862911. Accessed 22 Dec 2021
Stereotactic ablative radiotherapy for comprehensive treatment of 4–10 Oligometastatic tumors (SABR-COMET 10). Clin. Trials.gov NCT03721341. https://clinicaltrials.gov/ct2/show/NCT03721341. Accessed 22 Dec 2021
Trial of superiority of stereotactic body radiation therapy in patients with breast cancer (STEREOSTEIN). Clin. Trials.gov NCT02089100. https://clinicaltrials.gov/ct2/show/NCT02089100. Accessed 22 Dec 2021
Chao ST, Koyfman SA, Woody N, Angelov L, Soeder SL, Reddy CA, et al. Recursive partitioning analysis index is predictive for overall survival in patients undergoing spine stereotactic body radiation therapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2012;82:1738–43. https://doi.org/10.1016/j.ijrobp.2011.02.019.
Jensen G, Tang C, Hess KR, Bishop AJ, Pan HY, Li J, et al. Internal validation of the prognostic index for spine metastasis (PRISM) for stratifying survival in patients treated with spinal stereotactic radiosurgery. J Radiosurg SBRT. 2017;5:25–34. https://doi.org/10.1016/j.ijrobp.2017.02.085.
Zeng KL, Sahgal A, Tseng C-L, Myrehaug S, Soliman H, Detsky J, et al. Prognostic factors associated with surviving less than 3 months vs greater than 3 years specific to spine stereotactic body radiotherapy and late adverse events. Neurosurgery. 2021;88:971–9. https://doi.org/10.1093/neuros/nyaa583.
Palma DA, Louie AV, Rodrigues GB. New strategies in stereotactic radiotherapy for oligometastases. Clin Cancer Res. 2015;21:5198–204. https://doi.org/10.1158/1078-0432.CCR-15-0822.
Greco C, Pares O, Pimentel N, Louro V, Morales J, Nunes B, et al. Phenotype-oriented ablation of oligometastatic cancer with single dose radiation therapy. Int J Radiat Oncol Biol Phys. 2019;104:593–603. https://doi.org/10.1016/j.ijrobp.2019.02.033.
Thibault I, Chang EL, Sheehan J, Ahluwalia MS, Guckenberger M, Sohn MJ, et al. Response assessment after stereotactic body radiotherapy for spinal metastasis: a report from the SPIne response assessment in Neuro-Oncology (SPINO) group. Lancet Oncol. 2015;1:e595-603. https://doi.org/10.1016/S1470-2045(15)00166-7.
Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–17. https://doi.org/10.1007/s00330-011-2221-4.
Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren MV. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275–82.
Nakanishi K, Tanaka J, Nakaya Y, Maeda N, Sakamoto A, Nakayama A, et al. Whole-body MRI: detecting bone metastases from prostate cancer. Jpn J Radiol. 2022;40:229–44. https://doi.org/10.1007/s11604-021-01205-6.
Ording Müller LSO, Avenarius D, Olsen OE. High signal in bone marrow at diffusion-weighted imaging with body background suppression (DWIBS) in healthy children. Pediatr Radiol. 2011;41:221–6. https://doi.org/10.1007/s00247-010-1774-8.
Kwee TC, Takahara T, Ochiai R, Nievelstein RAJ, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008;18:1937–52. https://doi.org/10.1007/s00330-008-0968-z.
Ishiguchi H, Ito S, Kato K, Sakurai Y, Kawai H, Fujita N, et al. Diagnostic performance of 18 F-FDG PET/CT and whole-body diffusion-weighted imaging with background body suppression (DWIBS) in detection of lymph node and bone metastases from pediatric neuroblastoma. Ann Nucl Med. 2018;32:348–62. https://doi.org/10.1007/s12149-018-1254-z.
Sun W, Li M, Gu Y, Sun Z, Qiu Z, Zhou Y. Diagnostic value of whole-body DWI with background body suppression plus calculation of apparent diffusion coefficient at 3 T versus 18 F-FDG PET/CT for detection of bone metastases. AJR Am J Roentgenol. 2020;214:446–54. https://doi.org/10.2214/AJR.19.21656.
Sakurai Y, Kawai H, Iwano S, Ito S, Ogawa H, Naganawa S. Supplemental value of diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) technique to whole-body magnetic resonance imaging in detection of bone metastases from thyroid cancer. J Med Imaging Radiat Oncol. 2013;57:297–305. https://doi.org/10.1111/1754-9485.12020.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–17. https://doi.org/10.1148/rg.2018170108.
Ito K, Nakamura N, Shimizuguchi T, Ogawa H, Karasawa K. Appropriate endpoints for stereotactic body radiotherapy for bone metastasis: classification into five treatment groups. Rep Pract Oncol Radiother. 2020;25:150–3. https://doi.org/10.1016/j.rpor.2019.12.018.
Zelefsky MJ, Yamada Y, Greco C, Lis E, Schöder H, Lobaugh S, et al. Phase 3 multi-center, prospective, randomized trial comparing single-dose 24 Gy radiation therapy to a 3-fraction SBRT regimen in the treatment of Oligometastatic cancer. Int J Radiat Oncol Biol Phys. 2021;110:672–9. https://doi.org/10.1016/j.ijrobp.2021.01.004.
Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. J Spine. 2007;32:193–9. https://doi.org/10.1097/01.brs.0000251863.76595.a2.
Gibbs IC, Kamnerdsupaphon P, Ryu MR, Dodd R, Kiernan M, Chang SD, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–90. https://doi.org/10.1016/j.radonc.2006.11.023.
Sahgal A, Ames C, Chou D, Ma L, Huang K, Xu W, et al. Stereotactic body radiotherapy is effective salvage therapy for patients with prior radiation of spinal metastases. Int J Radiat Oncol Biol Phys. 2009;74:723–31. https://doi.org/10.1016/j.ijrobp.2008.09.020.
Patel VB, Wegner RE, Heron DE, Flickinger JC, Gerszten P, Burton SA. Comparison of whole versus partial vertebral body stereotactic body radiation therapy for spinal metastases. Technol Cancer Res Treat. 2012;11:105–15. https://doi.org/10.7785/tcrt.2012.500239.
Cox BW, Spratt DE, Lovelock M, Bilsky MH, Lis E, Ryu S, et al. International spine radiosurgery consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597-605. https://doi.org/10.1016/j.ijrobp.2012.03.009.
Singh R, Lehrer EJ, Dahshan B, Palmer J, Sahgal A, Gerszten PC, et al. Single fraction radiosurgery, fractionated radiosurgery, and conventional radiotherapy for spinal oligometastasis (SAFFRON): a systematic review and meta-analysis. Radiother Oncol. 2020;146:76–89. https://doi.org/10.1016/j.radonc.2020.01.030.
Soltys SG, Grimm J, Milano MT, Xue J, Sahgal A, Yorke E, et al. Stereotactic body radiation therapy for spinal metastases: tumor control probability analyses and recommended reporting standards. Int J Radiat Oncol Biol Phys. 2021;110:112–23. https://doi.org/10.1016/j.ijrobp.2020.11.021.
Glicksman RM, Tjong MC, Neves-Junior WFP, Spratt DE, Chua KLM, Mansouri A, et al. Stereotactic ablative radiotherapy for the management of spinal metastases: a review. JAMA Oncol. 2020;6:567–77. https://doi.org/10.1001/jamaoncol.2019.5351.
Hardcastle N, Bignell F, Nelms B, Siva S, Kneebone A, Lao L, et al. The challenge of planning vertebral body SBRT: optimizing target volume coverage. Med Dosim. 2020;45:302–7. https://doi.org/10.1016/j.meddos.2020.02.005.
Bishop AJ, Tao R, Rebueno NC, Christensen EN, Allen PK, Wang XA, et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int J Radiat Oncol Biol Phys. 2015;92:1016–26. https://doi.org/10.1016/j.ijrobp.2015.03.037.
Lovelock DM, Zhang Z, Jackson A, Keam J, Bekelman J, Bilsky M, et al. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int J Radiat Oncol Biol Phys. 2010;77:1282–7. https://doi.org/10.1016/j.ijrobp.2009.10.003.
Yamada Y, Katsoulakis E, Laufer I, Lovelock M, Barzilai O, McLaughlin LA, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42:E6. https://doi.org/10.3171/2016.9.FOCUS16369.
Al-Omair A, Masucci L, Masson-Cote L, Cambell M, Atenafu EG, Parent A, et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15:1413–9. https://doi.org/10.1093/neuonc/not101.
Sahgal A, Atenafu EG, Chao S, Al-Omair A, Boehling N, Balagamwala EH, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol. 2013;31:3426–31. https://doi.org/10.1200/JCO.2013.50.1411.
Sahgal A, Myrehaug SD, Siva S, Masucci GL, Maralani PJ, Brundage M, et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021;22:1023–33. https://doi.org/10.1016/S1470-2045(21)00196-0.
Ryu S, Deshmukh S, Timmerman RD, Movsas B, Gerszten PC, Yin FF, et al. Radiosurgery compared to external beam radiotherapy for localized spine metastasis: phase III results of NRG oncology/RTOG 0631. Int J Radiat Oncol. 2019;105:S2-3. https://doi.org/10.1016/j.ijrobp.2019.06.382.
Sahgal A, Weinberg V, Ma L, Chang E, Chao S, Muacevic A, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85:341–7. https://doi.org/10.1016/j.ijrobp.2012.05.007.
Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–101. https://doi.org/10.1118/1.3438081.
Kim DWN, Medin PM, Timmerman RD. Emphasis on repair, not just avoidance of injury, facilitates prudent stereotactic ablative radiotherapy. Semin Radiat Oncol. 2017;27:378–92. https://doi.org/10.1016/j.semradonc.2017.04.007.
Katsoulakis E, Jackson A, Cox B, Lovelock M, Yamada Y. A detailed dosimetric analysis of spinal cord tolerance in high-dose spine radiosurgery. Int J Radiat Oncol Biol Phys. 2017;99:598–607. https://doi.org/10.1016/j.ijrobp.2017.05.053.
Grimm J, Sahgal A, Soltys SG, Luxton G, Patel A, Herbert S, et al. Estimated risk level of unified stereotactic body radiation therapy dose tolerance limits for spinal cord. Semin Radiat Oncol. 2016;26:165–71. https://doi.org/10.1016/j.semradonc.2015.11.010.
Ghia AJ, Guha-Thakurta N, Hess K, Yang JN, Settle SH, Sharpe HJ, et al. Phase 1 study of spinal cord constraint relaxation with single session spine stereotactic radiosurgery in the primary management of patients with inoperable, previously unirradiated metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2018;102:1481–8. https://doi.org/10.1016/j.ijrobp.2018.07.2023.
Ito K, Furuya T, Shikama N, Nihei K, Tanaka H, Kumazaki Y, et al. A prospective multicentre feasibility study of stereotactic body radiotherapy in Japanese patients with spinal metastases. Jpn J Clin Oncol. 2019;49:999–1003. https://doi.org/10.1093/jjco/hyz130.
Ito K, Sugita S, Nakajima Y, Furuya T, Hiroaki O, Hayakawa S, et al. Phase 2 clinical trial of separation surgery followed by stereotactic body radiation therapy for metastatic epidural spinal cord compression. Int J Radiat Oncol Biol Phys. 2022;112:106–13. https://doi.org/10.1016/j.ijrobp.2021.07.1690.
Ito K, Ogawa H, Shimizuguchi T, Nihei K, Furuya T, Tanaka H, et al. Stereotactic body radiotherapy for spinal metastases: clinical experience in 134 cases from a single Japanese institution. Technol Cancer Res Treat. 2018;17:1533033818806472. https://doi.org/10.1177/1533033818806472.
Moussazadeh N, Lis E, Katsoulakis E, Kahn S, Svoboda M, DiStefano NM, et al. Five-year outcomes of high-dose single-fraction spinal stereotactic radiosurgery. Inter J Radiat Oncol Biol Phys. 2015;93:361–7. https://doi.org/10.1016/j.ijrobp.2015.05.035.
Funding
None.
Author information
Authors and Affiliations
Contributions
Author contributions to the study and manuscript preparation include the following. Conception and design: KI. Acquisition of data: NA. Analysis and interpretation of data: NA. Drafting the article: KI. Critically revising the article: none. Reviewed final version of the manuscript and approved it for submission: all the authors. Study supervision: none.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable because of a review article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ito, K., Nakajima, Y. & Ikuta, S. Stereotactic body radiotherapy for spinal oligometastases: a review on patient selection and the optimal methodology. Jpn J Radiol 40, 1017–1023 (2022). https://doi.org/10.1007/s11604-022-01277-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01277-y