Abstract
Purpose
To evaluate the high-resolution CT (HRCT) findings of pulmonary infections in patients with hematologic malignancy and compare them between patients with or without hematopoietic stem cell transplantation (HSCT).
Materials and methods
A total of 128 patients with hematologic malignancy and pulmonary infection were included in this study. The diagnoses of the patients consisted of bacterial pneumonia (37 non-HSCT cases and 14 HSCT cases), pneumocystis pneumonia (PCP) (29 non-HSCT cases and 11 HSCT cases), and fungal infection other than PCP (20 non-HSCT cases and 17 HSCT cases). Two chest radiologists retrospectively evaluated the HRCT criteria and compared them using chi-squared tests and a multiple logistic regression analysis.
Results
According to the multiple logistic regression analysis, nodules were an indicator in HSCT patients with PCP (p = 0.025; odds ratio, 5.8; 95% confidence interval, 1.2–26.6). The centrilobular distribution of nodules was the most frequent (n = 4, 36%) in HSCT patients with PCP. A mosaic pattern was an indicator of PCP in both HSCT and non-HSCT patients. There were no significant differences in other infections.
Conclusion
The mosaic pattern could be an indicator of PCP in both HSCT and non-HSCT patients. Nodules with centrilobular distribution might be relatively frequent HRCT findings of PCP in HSCT patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pulmonary infections are the common life-threatening complications in immunocompromised patients with hematologic malignancy. In addition, hematopoietic stem cell transplantation (HSCT) is performed to treat hematologic malignancy and is performed after high-dose chemotherapy or radiation therapy, which is a risk factor for severe opportunistic infections and pulmonary infections after HSCT [1, 2]. An early diagnosis of pulmonary infections is essential to develop an appropriate treatment strategy for these patients.
The differential diagnosis of pulmonary infections in immunocompromised patients could be established with the help of high-resolution CT (HRCT), especially for bacterial pneumonia, pneumocystis pneumonia (PCP), and fungal infection other than PCP [3]. However, some of the characteristic HRCT findings different according to the various immunocompromised statuses. The characteristic HRCT findings of pulmonary infection could be different between HSCT and non-HSCT patients with hematologic malignancy. To our knowledge, no previous studies have evaluated the differences in the HRCT findings of pulmonary infections between HSCT and non-HSCT patients. The purpose of this study was to evaluate and compare the HRCT findings of pulmonary infections (bacterial pneumonia, PCP, and fungal infection other than PCP) in patients with hematological malignancy who were managed with or without HSCT.
Materials and methods
The institutional review board of our institution approved this study. The requirement for informed consent was waived due to the retrospective design.
Patients
A total of 128 patients were included in this study from January 1990 to December 2015. There were 78 men and 50 women, and mean age was 49.6 (standard deviation [SD], 17.9; age range 5–77) years.
The diagnoses of the patients consisted of bacterial pneumonia (37 non-HSCT cases and 14 HSCT cases), PCP (29 non-HSCT cases and 11 HSCT cases), and fungal infection other than PCP (20 non-HSCT cases and 17 HSCT cases). Fungal infection other than PCP consisted of pulmonary aspergillosis and pulmonary candidiasis in this study. The exclusion criteria were as follows: (1) the presence of co-infection, (2) immunocompromised state due to a condition other than hematologic malignancy, and (3) other pulmonary abnormalities that could cause difficulty in evaluating HRCT (e.g., interstitial pneumonia, radiation pneumonitis, lung cancer, severe emphysema, bronchial asthma, and graft versus host disease). Diagnoses for infections were established by sputum culture (n = 30), bronchoalveolar lavage (BAL) or transbronchial biopsy (TBLB) (n = 32), serum marker (n = 35 including β-D-glucan > 31 pg/mL for PCP [4], n = 16; Aspergillus antigen, n = 19), blood culture (n = 22), autopsy (n = 7), urinary antigen test (n = 1), and surgical lung biopsy (n = 1).
The mean periods between transplantation and CT examination date were 482 (SD, 539; range 18–1652) days in bacterial pneumonia, 347 (SD, 309; 62–1224) days in PCP, and 263 (SD, 330; 13–1338) days in fungal infection other than PCP.
The purpose of this study was to evaluate the HRCT findings of pulmonary infections (bacterial pneumonia, PCP, and fungal infection other than PCP) in patients with hematologic malignancy and to compare them between HSCT and non-HSCT patients. First, we evaluated the difference between each infection in HSCT and non-HSCT patients. Next, we identified the significant indicator for the differentiation of each infection in HSCT or non-HSCT patients. Although the subjects recruited for this study were previously reported in another study that included 345 subjects and which compared HRCT findings among infectious diseases [3], this study was focused on cases with hematologic malignancy and performed with the specific goal of comparing the differences in the HRCT findings of pulmonary infections (bacterial pneumonia, PCP, and fungal infection other than PCP) between HSCT and non-HSCT patients. Therefore, the purpose of the current study differs from that of the previous report [3].
HRCT examinations
CT examinations at our institution were performed using one of the following CT scanners: TCT-900S (Canon Medical Systems Corporation), Somatom Plus 4, Volume Zoom, Somatom Definition, and Somatom Sensation 64 (Siemens Healthineers Systems). The CT scans were obtained at suspended end-inspiratory effort in the supine position without intravenous contrast material injection. Regarding examination by the TCT-900S, HRCT of the region showing abnormal findings was obtained at 2-mm collimation after obtaining conventional 10-mm collimation scans at contiguous 10 mm intervals. The CT images obtained by the TCT-900S scanner during the early period of data collection were viewed on hard copy films. As for the other multi-slice CT scanners, after contiguous 10-, 7-, or 5-mm section images were reconstructed through the chest, additional HRCT images consisting of 1 or 2 mm collimation were reconstructed at 1-, 2-, 5-, or 10-mm intervals through the abnormal findings. The scanning parameters were 120 or 140 kVp and 160–250 effective mAs for all CT scanners. All image data, with the exception of the TCT-900S data, were interfaced directly to our picture archiving and communication system (PACS) (ShadeQuest, Yokogawa Medical Solutions Corp.). The PACS displayed the image data on monitors (three monitors, 1280 × 1080 matrix, 8-bit viewable gray-scale) for blind reading. All images on the monitors or the hard copy films for the TCT-900 s were used to view both the lung (window width, 1500 or 1750 HU; window level, − 600 or − 700 HU) and mediastinum (window width, 250–400 HU; window level, 40–50 HU).
Interpretation of the HRCT images
The following HRCT findings were coded as present or absent: (a) consolidation (Con); (b) ground-glass attenuation (GGA); (c) crazy-paving pattern; (d) mosaic pattern; (e) nodules; (f) CT-halo sign; (g) tree-in-bud pattern; (h) bronchial wall thickening; (i) interlobular septal (ILS) thickening; (j) hilar or mediastinal lymph node (LN) enlargement; and (k) pleural effusion. Regarding the HRCT findings of (a), (b), (e) and the overall lesion, the extent of lesions within the entire lung field was graded (0 = 0%, 1 = 1–25%, 2 = 26–50%, 3 = 51–75%, and 4 = 76–100%).
According to GGA or Con, the distribution was classified as segmental, nonsegmental, or lobular. The nodules were classified as micro (< 3 mm), small (3–10 mm), or large (> 10 mm) according to their size and their distribution was also classified as centrilobular, perilymphatic, or random.
As for the overall lesion, the distribution was classified axially as central, peripheral, diffuse, or indeterminate and craniocaudally as upper, lower, diffuse, or indeterminate. Finally, one predominant HRCT pattern was recorded as follows: small nodule, large nodule, diffuse GGA, segmental GGA/Con, non-segmental GGA/Con, or bronchial wall or ILS thickening pattern.
Two chest radiologists (15 and 28 years of experience, respectively) evaluated the HRCT images in random order without any knowledge of the patients’ clinical information except for the fact that all patients had hematologic malignancies. Discordant results between the radiologists were resolved by consensus.
Statistical analysis
The mean age of the patients was calculated for each infection. The sex and HRCT finding patterns were compared between the HSCT and non-HSCT groups using a chi-squared test as an independent test for each infection. The extent of lesions was finalized as the average of the grades allocated by the two radiologists. The age and the extent of the HRCT findings were compared using a t test. Interobserver agreement between the two radiologists was calculated as the kappa value (κ) for the aforementioned HRCT findings from (a) to (k) and as the intraclass correlation coefficient (ICC) for the extent of the lesion, and was rated as follows: slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.00). Multiple logistic regression analyses were conducted to identify significant indicators for differentiation between each infection in HSCT and non-HSCT patients. In addition, multiple logistic regression analyses were also conducted to identify significant indicators for the differentiation of each infection from other infections, for example, between bacterial pneumonia and other infections (the combination of PCP and fungal infection other than PCP) in HSCT and non-HSCT cases. The forward selection (likelihood ratio) method was used for the multiple logistic regression analyses, and all variables. including parametric factors, were included. The area under the curve (AUC) of each model was calculated. The statistical analyses were performed using the SPSS software program (version 22.0, IBM). P values of < 0.05 were considered to indicate statistical significance.
Results
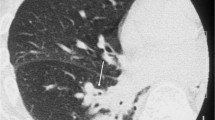
The HSCT patients were significantly younger than the non-HSCT patients for each of the three infections (p < 0.05) (Table 1). The interobserver agreement of the HRCT findings was slight to substantial (0.15–0.72). For PCP, nodules were more frequent in HSCT patients (55%) (Fig. 1) in comparison to non-HSCT patients (17%) (p = 0.027). The centrilobular distribution was the most frequent (36%) in HSCT patients with PCP. There were no significant differences in the findings of bacterial pneumonia and fungal infection other than PCP between HSCT patients and non-HSCT patients (Table 1). A multiple logistic regression analysis showed that nodules were an indicator for HSCT patients (p = 0.025; odds ratio [OR], 5.8; 95% confidence interval [CI] 1.2–26.6) with PCP.
A 59-year-old woman with pneumocystis pneumonia under treatment for malignant lymphoma after hematopoietic stem cell transplantation. A High-resolution CT shows ground-glass attenuation with small nodules with centrilobular distribution (arrows). B High-resolution CT shows small ground-glass nodules
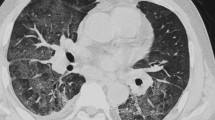
Among non-HSCT patients (Table 2), the following indicators for differentiation among the infectious groups were identified: the absence of a mosaic pattern (p = 0.006; OR, 4.5; 95% CI 1.5–13.3) and the presence of bronchial wall thickening (p = 0.008; OR, 3.8; 95% CI 1.4–10.1) for bacterial pneumonia; the presence of a mosaic pattern (p < 0.001; OR, 15.9; 95% CI 3.9–64.4) and the absence of nodules (p = 0.012; OR, 6.0; 95% CI 1.5–24.6) and bronchial wall thickening (p = 0.006; OR, 10.2; 95% CI 1.9–53.5) for PCP (Fig. 2); and the presence of nodules (p = 0.001; OR, 29.2; 95% CI 3.7–231.8) for fungal infection other than PCP.
The AUC values of the model for bacterial pneumonia, PCP, and fungal infection other than PCP in non-HSCT patients were 0.75, 0.92, and 0.78, respectively (Table 3).
Among HSCT patients (Table 4), the following indicators for differentiation among the infectious groups were identified: the presence of consolidation (p = 0.022; OR, 5.7; 95% CI 1.3–25.0) for bacterial pneumonia; the presence of a mosaic pattern (p = 0.005; OR, 11.2; 95% CI 2.1–60.1) for PCP; and the absence of GGA (p = 0.024; OR, 13.1; 95% CI 1.4–122.2) for fungal infection other than PCP. The mosaic pattern was detected as an indicator for PCP in both HSCT and non-HSCT patients.
The AUC values of the model for bacterial pneumonia, PCP, and fungal infection other than PCP in HSCT patients were 0.70, 0.72, and 0.66, respectively (Table 5).
Discussion
Our study showed that there are both similarities and differences in HRCT findings between HSCT patients and non-HSCT patients.
In PCP, nodules were observed significantly more frequently in HSCT patients than in non-HSCT patients. There were no significant differences in the HRCT findings of HSCT and non-HSCT patients in bacterial pneumonia and fungal infection other than PCP. On the other hand, the model for each infection between HSCT and non-HSCT patients was identified in this study. The AUC values of the model for PCP were the highest among these infections (0.92 in non-HSCT and 0.72 in HSCT).
In PCP, the mosaic pattern was an indicator in both HSCT and non-HSCT patients in our study. In addition, the absence of nodules was an indicator in non-HSCT patients. Previous studies reported that the mosaic pattern was frequently observed in PCP, while nodules were an unusual finding in PCP [3, 5,6,7]. Nodules have shown to represent granulomas and could be observed in human immunodeficiency virus (HIV) infection, and hematopoietic and solid malignancies [8]. The granulomatous reaction is related to host factors, including immune reconstitution disease and the recovery of partial immune efficiency following the interruption of corticosteroid treatment [9]. HSCT patients with PCP could show nodules because of their relatively strong immunosuppression and immune reconstitution state under treatment.
For bacterial pneumonia, the bronchial wall thickening was an indicator in HSCT patients and the consolidation was an indicator in non-HSCT patients. Bronchial wall thickening and consolidation are common characteristics of bacterial pneumonia [3, 10,11,12].
For fungal infection other than PCP, the nodules could be an indicator in non-HSCT patients. This result supports the findings of a previous study [3]. Invasive aspergillosis shows nodules with the halo sign in the early phase, and cavitary lesions with the air crescent sign in the late phase [13,14,15]. Nodules and cavitation are also common findings in cryptococcosis [16] though patients with cryptococcosis were not included in this study. Small nodules with centrilobular or random distribution are common in candidiasis [17, 18]. The absence of GGA could be an indicator in HSCT patients; however, GGA is a nonspecific finding and its sensitivity was low in the present study (35%).
The present study was associated with some limitations. First, this study was retrospective in nature; thus, the CT protocols and diagnostic procedures were diverse. However, the minimum CT protocols required to obtain the presented result were satisfied. Second, the reliability of the diagnosis may be controversial in patients without pathological confirmation. However, patients with infections due to other organisms or co-infections were excluded from our study, and diagnoses were strongly supported by serum markers or urinary antigen tests. Third, the interobserver agreement score was low of some criteria. The kappa values of mosaic pattern and nodules were 0.41 and 0.35, respectively. These results could affect the reliability of this study. These findings and ILS thickening could be overestimated by one radiologist. However, the discordant results between radiologists were resolved by consensus. The other criteria were not simple binary variables; thus, therefore, inter-individual variations may be noted. Forth, the patients with other infections such as cytomegalovirus pneumonia were not included in this study, because the number was limited. The purpose of this study was to focus on cases with hematologic malignancy based on the previous study, which identified the significant indicators especially for bacterial pneumonia, PCP, and fungal infection other than PCP among immunocompromised patients [3].
In conclusion, HRCT findings should be evaluated with consideration of the patient’s state irrespective of whether they underwent HSCT. In PCP, while the mosaic pattern could be the indicator of PCP in both HSCT and non-HSCT patients, nodules might be relatively frequent in HSCT patients than in non-HSCT patients.
References
Bondeelle L, Bergeron A. Managing pulmonary complications in allogeneic hematopoietic stem cell transplantation. Expert Rev Respir Med. 2019;13(1):105–19.
Coy DL, Ormazabal A, Godwin JD, Lalani T. Imaging evaluation of pulmonary and abdominal complications following hematopoietic stem cell transplantation. Radiographics. 2005;25(2):305–17 (Discussion 318).
Kunihiro Y, Tanaka N, Kawano R, Yujiri T, Kubo M, Ueda K, et al. Differential diagnosis of pulmonary infections in immunocompromised patients using high-resolution computed tomography. Eur Radiol. 2019;29(11):6089–99.
Tasaka S, Hasegawa N, Kobayashi S, Yamada W, Nishimura T, Takeuchi T, et al. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest. 2007;131(4):1173–80.
Boiselle PM, Crans CA Jr, Kaplan MA. The changing face of pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999;172(5):1301–9.
Fujii T, Nakamura T, Iwamoto A. Pneumocystis pneumonia in patients with HIV infection: clinical manifestations, laboratory findings, and radiological features. J Infect Chemother. 2007;13(1):1–7.
Kuhlman JE, Kavuru M, Fishman EK, Siegelman SS. Pneumocystis carinii pneumonia: spectrum of parenchymal CT findings. Radiology. 1990;175(3):711–4.
Hartel PH, Shilo K, Klassen-Fischer M, Neafie RC, Ozbudak IH, Galvin JR, et al. Granulomatous reaction to pneumocystis jirovecii: clinicopathologic review of 20 cases. Am J Surg Pathol. 2010;34(5):730–4.
Totet A, Duwat H, Daste G, Berry A, Escamilla R, Nevez G. Pneumocystis jirovecii genotypes and granulomatous pneumocystosis. Med Mal Infect. 2006;36(4):229–31.
Morikawa K, Okada F, Ando Y, Ishii R, Matsushita S, Ono A, et al. Meticillin-resistant staphylococcus aureus and meticillin-susceptible S. aureus pneumonia: comparison of clinical and thin-section CT findings. Br J Radiol. 2012;85(1014):168–75.
Okada F, Ando Y, Nakayama T, Tanoue S, Ishii R, Ono A, et al. Pulmonary thin-section CT findings in acute Moraxella catarrhalis pulmonary infection. Br J Radiol. 2011;84(1008):1109–14.
Okada F, Ando Y, Tanoue S, Ishii R, Matsushita S, Ono A, et al. Radiological findings in acute haemophilus influenzae pulmonary infection. Br J Radiol. 2012;85(1010):121–6.
Brodoefel H, Vogel M, Hebart H, Einsele H, Vonthein R, Claussen C, et al. Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis: kinetics of CT morphology and correlation with clinical findings and outcome. AJR Am J Roentgenol. 2006;187(2):404–13.
Kuhlman JE, Fishman EK, Burch PA, Karp JE, Zerhouni EA, Siegelman SS. CT of invasive pulmonary aspergillosis. AJR Am J Roentgenol. 1988;150(5):1015–20.
Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157(3):611–4.
Chang WC, Tzao C, Hsu HH, Lee SC, Huang KL, Tung HJ, et al. Pulmonary cryptococcosis: comparison of clinical and radiographic characteristics in immunocompetent and immunocompromised patients. Chest. 2006;129(2):333–40.
Tanaka N, Kunihiro Y, Yanagawa N. Infection in Immunocompromised Hosts: Imaging. J Thorac Imaging. 2018;33(5):306–21.
Franquet T, Muller NL, Lee KS, Oikonomou A, Flint JD. Pulmonary candidiasis after hematopoietic stem cell transplantation: thin-section CT findings. Radiology. 2005;236(1):332–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose with respect to this manuscript.
Ethical approval
The authors declare that they preserve ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kunihiro, Y., Tanaka, N., Kawano, R. et al. High-resolution CT findings of pulmonary infections in patients with hematologic malignancy: comparison between patients with or without hematopoietic stem cell transplantation. Jpn J Radiol 40, 791–799 (2022). https://doi.org/10.1007/s11604-022-01260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-022-01260-7