Abstract
Objective
We evaluated the usefulness of fat-suppressed three-dimensional T1-weighted volume isotropic turbo spin-echo acquisition (FS 3D T1W-VISTA) imaging for the evaluation of the ectopic posterior pituitary gland (EPPG).
Materials and methods
This retrospective study included 9 patients with EPPG due to causes other than tumor. All underwent sagittal two-dimensional (2D) T1W-, FS 3D T1W-VISTA- (VISTA), and 3D T2W-driven equilibrium radiofrequency reset pulse (DRIVE) imaging. Two radiologists independently reviewed the 2D T1W- and VISTA images for their image quality and for visualization of the EPPG and of pituitary stalk transection. DRIVE findings were used as the reference standard for pituitary stalk transection. Interobserver and intermodality agreements were evaluated with the kappa (κ) coefficient. The mean grade assigned to the 2D T1W- and the VISTA imaging technique for visualization of the EPPG was assessed by the Mann–Whitney U test.
Results
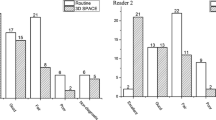
Interobserver agreement for visualization of the EPPG on 2D T1W- and VISTA images was excellent (κ = 0.82 and κ = 1.00, respectively). The mean grade for EPPG visualization was significantly higher for VISTA- than 2D T1W images (p = 0.0039).
Conclusion
FS 3D T1W-VISTA imaging is useful for the evaluation of EPPG.
A secondary abstract
Conventional MRI yields insufficient information for the evaluation of the ectopic posterior pituitary gland (EPPG). The visualization of the EPPG was significantly higher for fat-suppressed three-dimensional T1-weighted volume isotropic turbo spin-echo acquisition (FS 3D T1W-VISTA) than 2D T1W images. FS 3D T1W-VISTA imaging is useful for the evaluation of the EPPG.


Similar content being viewed by others
Abbreviations
- EPPG:
-
Ectopic posterior pituitary gland
- FS 3D T1W-VISTA:
-
Fat-suppressed three-dimensional T1-weighted volume isotropic turbo spin-echo acquisition
- DRIVE:
-
Driven equilibrium radiofrequency reset pulse
References
Di Iorgi N, Allegri AEM, Napoli F, Bertelli E, Olivieri I, Rossi A, et al. The use of neuroimaging for assessing disorders of pituitary development. Clin Endocrinol. 2012;76:161–76.
Maghnie M, Lindberg A, Koltowska-Häggström M, Ranke MB. Magnetic resonance imaging of CNS in 15,043 children with GH deficiency in KIGS (Pfizer International Growth Database). Eur J Endocrinol. 2013;168:211–7.
Di Iorgi N, Morana G, Allegri AEM, Napoli F, Gastaldi R, Calcagno A, et al. Classical and non-classical causes of GH deficiency in the peadiatric age. Best Pract Res Clin Endocrinol Metab. 2016;30:705–36.
Fujisawa I, Asato R, Kawata M, Sano Y, Nakao K, Yamada T, et al. Hyperintense signal of the posterior pituitary on T1-weighted MR images: An experimental study. J Comput Assist Tomogr. 1989;3(3):371–7.
Sato N, Tanaka S, Tateno M, Ohya N, Takata K, Endo K. Origin of posterior pituitary high intensity on T1-weighted magnetic resonance imaging. Immunohistochemical, electron microscopic, and magnetic resonance studies of posterior pituitary lobe of dehydrated rabbits. Invest Radiol. 1995;30(10):567–71.
Colombo N, Berry I, Kucharczyk J, Kucharczyk W, de Groot J, Larson T, et al. Posterior pituitary gland: appearance on MR images in normal and pathologic states. Radiology. 1987;165(2):481–5.
Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, et al. Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology. 1987;165(2):487–9.
Fujisawa I, Nishimura K, Asato R, Togashi K, Itoh K, Noma S, et al. Posterior lobe of the pituitary in diabetes insipidus: MR findings. J Comput Assist Tomogr. 1987;11:221–5.
Kaufman BA, Kaufman B, Mapstone TB. Pituitary stalk agenesis: magnetic resonance imaging of ‘ectopic posterior lobe’ with surgical correlation. Pediatr Neurosci. 1988;14:140–4.
Maintz D, Benz-Bohm G, Gindele A, Schönau E, Pfäffle R, Lackner K. Posterior pituitary ectopia: another hint toward a genetic etiology. AJNR Am J Neuroradiol. 2000;21:1116–8.
Kyriacou V, Mavridou Ch, Bintoudi A, Tzikos F, Kotziamani N, Tsitouridis I. Pituitary stalk interruption syndrome: the role of MRI and review of the literature. Neuroradiol J. 2010;23(5):607–12.
Chen S, Léger J, Garel C, Hassan M, Czernichow P. Growth hormone deficiency with ectopic neurohypophysis: anatomical variations and relationship between the visibility of the pituitary stalk asserted by magnetic resonance imaging and anterior pituitary function. J Clin Endocrinol Metab. 1999;84(7):2408–13.
Argyropoulou MI, Kiortsis DN. MRI of the hypothalamic-pituitary axis in children. Pediatr Radiol. 2005;35(11):1045–55.
Satogami N, Miki Y, Koyama T, Kataoka M, Togashi K. Normal pituitary stalk: high-resolution MR imaging at 3T. AJNR Am J Neuroradiol. 2010;31(2):355–9.
Sato N, Ishizaka H, Matsumoto M, Matsubara K, Tsushima Y, Tomioka K. MR detectability of posterior pituitary high signal and direction of frequency encoding gradient. J Comput Assist Tomogr. 1991;15(3):355–8.
Bapst B, Amegnizin JL, Vignaud A, Kauv P, Maraval A, Kalsoum E, et al. Post-contrast 3D T1-weighted TSE MR sequences (SPACE, CUBE, VISTA/BRAINVIEW, isoFSE, 3D MVOX): technical aspects and clinical applications. J Neuroradiol. 2020;S0150–9861(20):30111–5.
Haneda J, Ishikawa K, Okamoto K. Better continuity of the facial nerve demonstrated in the temporal bone on three-dimensional T1-weighted imaging with volume isotropic turbo spin echo acquisition than that with fast field echo at 3.0 tesla MRI. J Med Imaging Radiat Oncol. 2019;63(6):745–50.
Weishaupt D, Kochli VD, Marincek B. 13 MR artifacts. In: Weishaupt D, Kochli VD, Marincek B, editors. How does MRI work. 2nd ed. Berlin, Heidelberg: Springer-Verlag; 2008. p. 129–37.
Arslan A, Karaarslan E, Dinçer A. High intensity signal of the posterior pituitary. A study with horizontal direction of frequency-encoding and fat suppression MR techniques. Acta Radiol. 1999;40(2):142–5.
Mark LP, Haughton VM, Hendrix LE, Daniels DL, Williams AL, Czervionke LF, et al. High-intensity signals within the posterior pituitary fossa: a study with fat-suppression MR techniques. AJNR Am J Neuroradiol. 1991;12(3):529–32.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Dolezal O, Dwyer MG, Horakova D, Havrdova E, Minagar A, Balachandran S, et al. Detection of cortical lesions is dependent on choice of slice thickness in patients with multiple sclerosis. Int Rev Neurobiol. 2007;79:475–89.
Molyneux PD, Tubridy N, Parker GJ, Barker GJ, MacManus DG, Tofts PS, et al. The effect of section thickness on MR lesion detection and quantification in multiple sclerosis. AJNR Am J Neuroradiol. 1998;19(9):1715–20.
Bink A, Schmitt M, Gaa J, Mugler JP 3rd, Lanfermann H, Zanella FE. Detection of lesions in multiple sclerosis by 2D FLAIR and single-slab 3D FLAIR sequences at 3.0 T: Initial results. Eur Radiol. 2006;16(5):1104–10.
Ginat DT, Meyers SP. Intracranial lesions with high signal intensity on T1-weighted MR images: differential diagnosis. Radiographics. 2012;32:499–516.
Mark LP, Haughton VM, Hendrix LE, Daniels DL, Williams AL, Czervionke LF, et al. High-intensity signals within the posterior pituitary fossa: a study with fat suppression MR techniques. AJNR Am J Neuroradiol. 1991;2:529–32.
Lin Z, Zhang X, Guo L, Wang K, Jiang Y, Hu X, et al. Clinical feasibility study of 3D intracranial magnetic resonance angiography using compressed sensing. J Magn Reson Imaging. 2019;50(6):1843–51.
El Sanharawi I, Tzarouchi L, Cardoen L, Martinerie L, Leger J, Carel JC, et al. High-resolution heavily T2-weighted magnetic resonance imaging for evaluation of the pituitary stalk in children with ectopic neurohypophysis. Pediatr Radiol. 2017;47(5):599–605.
Funding
Not applicable. There is no funding to report for this submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Not applicable. We have no conflict of interest to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University of Miyazaki Hospital. Informed patient consent was waived.
Consent to participate
This retrospective study was reviewed and approved by our institutional review board. Informed patient consent was waived.
Consent for publication (include appropriate statements)
All authors of this submission approved the version to be published. All authors have understood the journal's licensing policy.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Azuma, M., Kadota, Y., Matsuyama, M. et al. 3D fat-suppressed T1-weighted volume isotropic turbo spin-echo acquisition (VISTA) imaging for the evaluation of the ectopic posterior pituitary gland. Jpn J Radiol 39, 564–570 (2021). https://doi.org/10.1007/s11604-020-01076-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-01076-3