Abstract
Objective
Oral squamous cell carcinoma (OSCC) is the most common malignant tumor of the head and neck, but its occurrence and progression mechanisms remain unclear. In addition-there is a lack of effective targeting drugs. The second major subunit of DNA polymerase (POLE2) catalyzes the prolongation of new strand replication and modifies exonuclease domain activity. Our previous study found that POLE2 was associated with OSCC progression, but the mechanism remains unclear.
Methods
The expression of POLE2 in OSCC tissues was detected using immunological assays. Mann-Whitney U analysis was used to investigate the relationship between POLE2 gene expression and tumor classification and prognosis of OSCC. POLE2 expression was inhibited in OSCC cells, and the effects of gene and protein expression were detected using RT-PCR and Western blotting. The POLE2 knockout model was constructed by transfecting a lentiviral vector. Cell proliferation, apoptosis, and migration were detected using various assays including colony formation, MTT, flow cytometry, wound healing assay, Transwell assay, and the Human Apoptosis Antibody Array. The animal model of OSCC was established by subcutaneous injection of transfected HN6 into 4-week-old female nude mice. After 30 days, tumors were removed under anesthesia and tumor weight and dimension were recorded. Tumor cell proliferation was analyzed using Ki67 staining.
Results
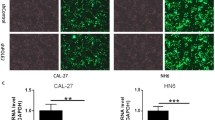
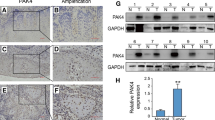
POLE2 gene levels were significantly higher in the OSCC tissues than in the normal tissues. In addition, POLE2 gene levels were statistically correlated with tumor classification and prognosis. Silencing POLE2 inhibited the proliferation of oral cancer cells and promoted apoptosis in vitro. Animal experiments also supported a positive correlation between POLE2 and OSCC tumor formation. We further demonstrated that POLE2 could upregulate the expression of apoptosis-related proteins such as caspase-3, CD40, CD40L, DR6, Fas, IGFBP-6, p21, and SMAC. In addition, POLE2 regulated OSCC development by inhibiting the PI3K/AKT signaling pathway.
Conclusion
POLE2 is closely related to the progression of OSCC. Thus, POLE2 may be a potential target for OSCC treatment.
Similar content being viewed by others
References
Bavle RM, Venugopal R, Konda P, et al. Molecular Classification of Oral Squamous Cell Carcinoma. J Clin Diagn Res, 2016,10(9):ZE18–ZE21
Chen W, Zheng R, Baade RD, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016,66(2):115–132
Almangush A, Makitie AA, Triantafyllou A, et al. Staging and grading of oral squamous cell carcinoma: An update, Oral Oncol, 2020,107:104799
Zhang WB, Wang Y, Mao C, et al. Oral Squamous Cell Carcinoma with Metastasis to the Parotid Lymph Node. Chin J Dent Res, 2019,22(3):175–179
Briggs S, Tomlinson I. Germline and somatic polymerase epsilon and delta mutations define a new class of hypermutated colorectal and endometrial cancers. J Pathol, 2013,230(2):148–153
Frugoni F, Dobbs K, Felgentreff K, et al. A novel mutation in the POLE2 gene causing combined immunodeficiency. J Allergy Clin Immunol, 2016,137(2):635–638.e1
Spier I, Holzapfel S, Altmuller J, et al. Frequency and phenotypic spectrum of germline mutations in POLE and seven other polymerase genes in 266 patients with colorectal adenomas and carcinomas. Int J Cancer, 2015,137(2):320–331
Zekri AR, Hassan ZK, Bahnassy AA, et al. Differentially expressed genes in metastatic advanced Egyptian bladder cancer. Asian Pac J Cancer Prev, 2015,16(8):3543–3549
Liu D, Zhang XX, Xi BX, et al. Sine oculis homeobox homolog 1 promotes DNA replication and cell proliferation in cervical cancer. Int J Oncol, 2014,45(3):1232–1240
Hartmann E, Fernandez V, Moreno V, et al. Five-gene model to predict survival in mantle-cell lymphoma using frozen or formalin-fixed, paraffin-embedded tissue. J Clin Oncol, 2008,26(30):4966–4972
Wu Z, Wang YM, Dai Y, et al. POLE2 Serves as a Prognostic Biomarker and Is Associated with Immune Infiltration in Squamous Cell Lung Cancer. Med Sci Monit, 2020,26:e921430
Li J, Wang J, Yu J, et al. Knockdown of POLE2 expression suppresses lung adenocarcinoma cell malignant phenotypes in vitro, Oncol Rep, 2018,40(5):2477–2486
Wu X, Deng Y. Bax and BH3-domain-only proteins in p53-mediated apoptosis, Front Biosci, 2002,7:d151–156
Jurmeister P, Bockmayr M, et al. Immunohistochemical analysis of Bcl-2, nuclear S100A4, MITF and Ki67 for risk stratification of early-stage melanoma - A combined IHC score for melanoma risk stratification. J Dtsch Dermatol Ges, 2019,17(8):800–808
Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst), 2016,42:63–71
Liu C, Vojnovic D, Kochevar IE, et al. UV-A Irradiation Activates Nrf2-Regulated Antioxidant Defense and Induces p53/Caspase3-Dependent Apoptosis in Corneal Endothelial Cells. Invest Ophthalmol Vis Sci, 2016,57(4):2319–2327
Matsumura Y, Hiraoka K, Ishikawa K, et al. CD40 Expression in Human Esophageal Squamous Cell Carcinoma Is Associated with Tumor Progression and Lymph Node Metastasis. Anticancer Res, 2016,36(9):4467–4475
Xue LY, Ren LQ, Luo W, et al. Expression of Fas, Fas ligand, Fas-associated death domain protein, caspase 8 and mutant P53 protein in esophageal squamous cell carcinoma. Zhonghua Yi Xue Za Zhi (Chinese), 2007,87(3):150–154
Qiao D, Xu J, Le C, et al. Insulin-like growth factor binding protein 5 (IGFBP5) mediates methamphetamine-induced dopaminergic neuron apoptosis. Toxicol Lett, 2014,230(3):444–453
Xu Y, Zhou L, Huang J, et al. Role of Smac in determining the chemotherapeutic response of esophageal squamous cell carcinoma, Clin Cancer Res, 2011,17(16):5412–5422
Wei J, Wu J, Xu W, et al. Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3K/AKT/HIF-1α signaling pathway. Cell Death Dis, 2018,9(6):599
Carlos de Vicente J, Herrero-Zapatero A, Fresno MF, et al. Expression of cyclin D1 and Ki-67 in squamous cell carcinoma of the oral cavity: clinicopathological and prognostic significance. Oral Oncol, 2002,38(3):301–308
Walter V, Yin X, Wilkerson MD, et al. Correction: Molecular Subtypes in Head and Neck Cancer Exhibit Distinct Patterns of Chromosomal Gain and Loss of Canonical Cancer Genes. PLoS One, 2018,13(3): e0194674
Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science, 2004,304(5670):55
Xu S, Ge J, Zhang Z, et al. MiR-129 inhibits cell proliferation and metastasis by targeting ETS1 via PI3K/AKT/mTOR pathway in prostate cancer. Biomed Pharmacother, 2017,96:634–641
Acknowledgements
We would like to thank the Department of Imaging, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology for the data support of this retrospective study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors declare that they have no direct conflicts of interest.
Additional information
This study was supported by grants from the National Natural Science Foundation of China (No. 82203418) and the Natural Science Foundation of Hubei Province (No. 2022CFB286 and No. 2021CFB589).
Rights and permissions
About this article
Cite this article
Sun, My., Wang, L. & Shen, Zy. POLE2 Regulates Apoptosis of Oral Squamous Cell Carcinoma Cells through the PI3K/AKT Signaling Pathway. CURR MED SCI 43, 1162–1172 (2023). https://doi.org/10.1007/s11596-023-2813-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2813-7