Abstract
Objective
The combination of stereotactic body radiation therapy (SBRT) and immune checkpoint inhibitors (ICIs) is actively being explored in advanced non-small-cell lung cancer (NSCLC) patients. However, little is known about the optimal fractionation and radiotherapy target lesions in this scenario. This study investigated the effect of SBRT on diverse organ lesions and radiotherapy dose fractionation regimens on the prognosis of advanced NSCLC patients receiving ICIs.
Methods
The medical records of advanced NSCLC patients consecutively treated with ICIs and SBRT were retrospectively reviewed at our institution from Dec. 2015 to Sep. 2021. Patients were grouped according to radiation sites. Progression-free survival (PFS) and overall survival (OS) were recorded using the Kaplan-Meier method and compared between different treatment groups using the log-rank (Mantel-Cox) test.
Results
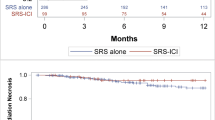
A total of 124 advanced NSCLC patients receiving ICIs combined with SBRT were identified in this study. Radiation sites included lung lesions (lung group, n=43), bone metastases (bone group, n=24), and brain metastases (brain group, n=57). Compared with the brain group, the mean PFS (mPFS) in the lung group was significantly prolonged by 13.3 months (8.5 months vs. 21.8 months, HR=0.51, 95%CI: 0.28–0.92, P=0.0195), and that in the bone group prolonged by 9.5 months with a 43% reduction in the risk of disease progression (8.5 months vs. 18.0 months, HR=0.57, 95%CI: 0.29–1.13, P=0.1095). The mPFS in the lung group was prolonged by 3.8 months as compared with that in the bone group. The mean OS (mOS) in the lung and bone groups was longer than that of the brain group, and the risk of death decreased by up to 60% in the lung and bone groups as compared with that of the brain group. When SBRT was concurrently given with ICIs, the mPFS in the lung and brain groups were significantly longer than that of the bone group (29.6 months vs. 16.5 months vs. 12.1 months). When SBRT with 8–12 Gy per fraction was combined with ICIs, the mPFS in the lung group was significantly prolonged as compared with that of the bone and brain groups (25.4 months vs. 15.2 months vs. 12.0 months). Among patients receiving SBRT on lung lesions and brain metastases, the mPFS in the concurrent group was longer than that of the SBRT→ICIs group (29.6 months vs. 11.4 months, P=0.0003 and 12.1 months vs. 8.9 months, P=0.2559). Among patients receiving SBRT with <8 Gy and 8–12 Gy per fraction, the mPFS in the concurrent group was also longer than that of the SBRT→ICIs group (20.1 months vs. 5.3 months, P=0.0033 and 24.0 months vs. 13.4 months, P=0.1311). The disease control rates of the lung, bone, and brain groups were 90.7%, 83.3%, and 70.1%, respectively.
Conclusion
The study demonstrated that the addition of SBRT on lung lesions versus bone and brain metastases to ICIs improved the prognosis in advanced NSCLC patients. This improvement was related to the sequence of radiotherapy combined with ICIs and the radiotherapy fractionation regimens. Dose fractionation regimens of 8–12 Gy per fraction and lung lesions as radiotherapy targets might be the appropriate choice for advanced NSCLC patients receiving ICIs combined with SBRT.
Similar content being viewed by others
References
Thai AA, Solomon BJ, Sequist LV, et al. Lung cancer. The Lancet, 2021,398(10299):535–554
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 2021,71(3):209–249
Gridelli C, Rossi A, Carbone DP, et al. Non-small-cell lung cancer. Nat Rev Dis Primers, 2015,1:15009
Chen VW, Ruiz BA, Hsieh MC, et al. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer, 2014,120 Suppl 23:3781–3792
Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol, 2016,11(1):39–51
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol, 2020,20(11):651–668
Yang CY, Yang JCH, Yang PC. Precision Management of Advanced Non-Small Cell Lung Cancer. Annu Rev Med, 2020,71(1):117–136
Chaft JE, Rimner A, Weder W, et al. Evolution of systemic therapy for stages I–III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol, 2021,18(9):547–557
Zhou C, Wang J, Wang B, et al. Chinese Experts Consensus on Immune Checkpoint Inhibitors for Non-small Cell Lung Cancer (2020 Version). Zhongguo Fei Ai Za Zhi (Chinese), 2021,24(4):217–235
Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab With or Without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol, 2020,6(5):661–674
Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol, 2021,22(2):198–211
Morad G, Helmink BA, Sharma P, et al. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell, 2021,184(21):5309–5337
Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol, 2021,18(10):625–644
Yi M, Zheng X, Niu M, et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer, 2022,21(1):28
Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med, 2018,24(5):541–550
Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res, 2017,5(5):417–424
Osorio JC, Arbour KC, Le DT, et al. Lesion-Level Response Dynamics to Programmed Cell Death Protein (PD-1) Blockade. J Clin Oncol, 2019,37(36):3546–3555
Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol, 2014,32(26):2847–2854
Diamant A, Heng VJ, Chatterjee A, et al. Comparing local control and distant metastasis in NSCLC patients between CyberKnife and conventional SBRT. Radiother Oncol, 2020,144:201–208
Schneider BJ, Daly ME, Kennedy EB, et al. Stereotactic Body Radiotherapy for Early-Stage Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology Evidence-Based Guideline. J Clin Oncol, 2018,36(7):710–719
Rieber J, Streblow J, Uhlmann L, et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases-A pooled analysis of the German working group “stereotactic radiotherapy”. Lung Cancer, 2016,97:51–58
Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. The Lancet, 2019,393(10185):2051–2058
Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol, 2020,38(25):2830–2838
Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst, 1979,63(5):1229–1235
Song CW, Glatstein E, Marks LB, et al. Biological Principles of Stereotactic Body Radiation Therapy (SBRT) and Stereotactic Radiation Surgery (SRS): Indirect Cell Death. Int J Radiat Oncol Biol Phys, 2021,110(1):21–34
Vanpouille-Box C, Formenti SC, Demaria S. Toward Precision Radiotherapy for Use with Immune Checkpoint Blockers. Clin Cancer Res, 2018,24(2):259–265
Marciscano AE, Haimovitz-Friedman A, Lee P, et al. Immunomodulatory Effects of Stereotactic Body Radiation Therapy: Preclinical Insights and Clinical Opportunities. Int J Radiat Oncol Biol Phys, 2021,110(1):35–52
Golden EB, Formenti SC. Is tumor (R)ejection by the immune system the “5th R” of radiobiology? Oncoimmunology, 2014,3(1):e28133
Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys, 2014,88(2):254–262
Lumniczky K, Safrany G. The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer Lett, 2015,356(1):114–125
Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin, 2017,67(1):65–85
Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest, 2014,124(2):687–695
Joseph RW, Elassaiss-Schaap J, Kefford R, et al. Baseline Tumor Size Is an Independent Prognostic Factor for Overall Survival in Patients with Melanoma Treated with Pembrolizumab. Clin Cancer Res, 2018,24(20):4960–4967
Robert C, Ribas A, Hamid O, et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients With Metastatic Melanoma. J Clin Oncol, 2018,36(17):1668–1674
Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature, 2017,545(7652):60–65
Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol, 2017,18(7):895–903
Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol, 2016,13(8):516–524
Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC—an Update From the PACIFIC Trial. J Thoracic Oncol, 2021,16(5):860–867
Dall’Olio FG, Marabelle A, Caramella C, et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat Rev Clin Oncol, 2022,19(2):75–90
Rodriguez-Ruiz ME, Sanmamed MF, Serrano-Mendioroz I, et al. Consolidating Radiotherapy with Immunotherapy. Clin Cancer Res, 2021,27(20):5443–5445
Theelen W, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol, 2019,5(9):1276–1282
Welsh J, Menon H, Chen D, et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I / II trial. J Immunother Cancer, 2020,8(2):e001001
Nikitas J, Roach M, Robinson C, et al. Treatment of oligometastatic lung cancer with brain metastases using stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT). Clin Transl Radiat Oncol, 2020,21:32–35
Chen L, Douglass J, Kleinberg L, et al. Concurrent Immune Checkpoint Inhibitors and Stereotactic Radiosurgery for Brain Metastases in Non-Small Cell Lung Cancer, Melanoma, and Renal Cell Carcinoma. Int J Radiat Oncol Biol Phys, 2018,100(4):916–925
Schapira E, Hubbeling H, Yeap BY, et al. Improved Overall Survival and Locoregional Disease Control With Concurrent PD-1 Pathway Inhibitors and Stereotactic Radiosurgery for Lung Cancer Patients With Brain Metastases. Int J Radiat Oncol Biol Phys, 2018,101(3):624–629
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009, 45(2):228–247
Chen Y, Gao M, Huang Z, et al. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol, 2020,13(1):105
Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med, 2021,9(5):467–475
Foster CC, Sher DJ, Rusthoven CG, et al. Overall survival according to immunotherapy and radiation treatment for metastatic non-small-cell lung cancer: a National Cancer Database analysis. Radiat Oncol, 2019,14(1):18
Wei J, Montalvo-Ortiz W, Yu L, et al. Sequence of αPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol, 2021,6(58):eabg0117
Samstein R, Rimner A, Barker CA, et al. Combined Immune Checkpoint Blockade and Radiation Therapy: Timing and Dose Fractionation Associated with Greatest Survival Duration Among Over 750 Treated Patients. Int J Radiat Oncol Biol Phys, 2017,99(2):S129–S130
Chmura SJ, Bestvina CM, Karrison TG, et al. Safety and Efficacy of a Randomized Phase I Trial to Evaluate Concurrent or Sequential Ipilimumab, Nivolumab, and Stereotactic Body Radiotherapy in Patients with Stage IV Non-small Cell Lung Cancer (COSINR Study). Int J Radiat Oncol Biol Phys, 2020,108(3):S72
Turchan WT, Pitroda SP, Weichselbaum RR. Radiotherapy and Immunotherapy Combinations in the Treatment of Patients with Metastatic Disease: Current Status and Future Focus. Clin Cancer Res, 2021,27(19):5188–5194
Lehrer EJ, Peterson J, Brown PD, et al. Treatment of brain metastases with stereotactic radiosurgery and immune checkpoint inhibitors: An international meta-analysis of individual patient data. Radiother Oncol, 2019,130:104–112
Deng L, Liang H, Xu M, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity, 2014,41(5):843–852
Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun, 2017,8:15618
Formenti SC. Optimizing Dose Per Fraction: A New Chapter in the Story of the Abscopal Effect? Int J Radiat Oncol Biol Phys, 2017,99(3):677–679
Vanpouille-Box C, Formenti SC, Demaria S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology, 2017,6(9):e1339857
Anscher MS, Arora S, Weinstock C, et al. Association of Radiation Therapy With Risk of Adverse Events in Patients Receiving Immunotherapy: A Pooled Analysis of Trials in the US Food and Drug Administration Database. JAMA Oncol, 2022,8(2):232–240
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest Statement
We declared that we had no conflicts of interest.
Author Gang WU is a member of the Editorial Board for Current Medical Science. Author Rui MENG is a member of Youth Editorial Committee for Current Medical Science. The paper was handled by the other editors and has undergone rigorous peer review process. Authors Gang WU and Rui MENG were not involved in the journal’s review of, or decisions related to, this manuscript.
Rights and permissions
About this article
Cite this article
Zhu, Kk., Wei, Jl., Xu, Yh. et al. Effect of Stereotactic Body Radiation Therapy on Diverse Organ Lesions in Advanced Non-Small Cell Lung Cancer Patients Receiving Immune Checkpoint Inhibitors. CURR MED SCI 43, 344–359 (2023). https://doi.org/10.1007/s11596-023-2702-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-023-2702-0