Abstract
Objective
Cytogenetic abnormalities have been proven to be the most valuable parameter for risk stratification of childhood acute lymphoblastic leukemia (ALL). However, studies on the prevalence of cytogenetic abnormalities and their correlation to clinical features in Chinese pediatric patients are limited, especially large-scale studies.
Methods
We collected the cytogenetics and clinical data of 1541 children newly diagnosed with ALL between 2001 and 2014 in four Chinese hospitals, and retrospectively analyzed their clinical features, prognosis and risk factors associated with pediatric ALL.
Results
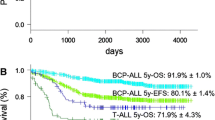
All of these patients had karyotyping results, and some of them were tested for fusion genes by fluorescence in situ hybridization or reverse-transcription polymerase chain reaction. Overall, 930 cases (60.4%) had abnormal cytogenetics in this study, mainly including high hyperdiploidy (HHD, n=276, 17.9%), hypodiploidy (n=74, 4.8%), t(12;21)/TEL-AML1 (n=260, 16.9%), t(1;19)/E2A-PBX1 (n=72, 4.7%), t(9;22)/BCR-ABL (n=64, 4.2%), and t(v;11q23)/MLL rearrangements (n=40, 2.6%). The distribution of each cytogenetic abnormality was correlated with gender, age, white blood cell count at diagnosis, and immunophenotype. In addition, multivariate analysis suggested that t(v;11q23)/MLL rearrangements (OR: 2.317, 95%CI: 1.219–3.748, P=0.008) and t(9;22)/BCR-ABL (OR: 2.519, 95%CI: 1.59–3.992, P<0.001) were independent risk factors for a lower event-free survival (EFS) rate in children with ALL, while HHD (OR: 0.638, 95%CI: 0.455–0.894, P=0.009) and t(12;21)/TEL-AML1 (OR: 0.486, 95%CI: 0.333–0.707, P<0.001) were independent factors of a favorable EFS.
Conclusion
The cytogenetic characteristics presented in our study resembled other research groups, emphasizing the important role of cytogenetic and molecular genetic classification in ALL, especially in B-ALL.
Similar content being viewed by others
References
Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet (London, England), 2020,395(10230):1146–1162
Martinez-Climent JA. Molecular cytogenetics of childhood hematological malignancies. Leukemia, 1997, 11(12):1999–2021
Harrison CJ, Haas O, Harbott J, et al. Detection of prognostically relevant genetic abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: recommendations from the Biology and Diagnosis Committee of the International Berlin-Frankfürt-Münster study group. Br J Haematol, 2010, 151(2):132–142
Moorman AV, Ensor HM, Richards SM, et al. Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol, 2010,11(5):429–438
Teachey DT, Pui CH. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol, 2019,20(3):e142–e54
Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol, 2009, 27(31):5175–5181
Kato M, Ishimaru S, Seki M, et al. Long-term outcome of 6-month maintenance chemotherapy for acute lymphoblastic leukemia in children. Leukemia, 2017, 31(3):580–584
Pui CH, Sandlund JT, Pei D, et al. Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA, 2003, 290(15):2001–2007
Aldrich MC, Zhang L, Wiemels JL, et al. Cytogenetics of Hispanic and White children with acute lymphoblastic leukemia in California. Cancer Epidemiol Biomarkers Prev, 2006,15(3):578–581
Ariffin H, Chen SP, Kwok CS, Quah T-C, Lin H-P, Yeoh AEJ. Ethnic differences in the frequency of subtypes of childhood acute lymphoblastic leukemia: results of the Malaysia-Singapore Leukemia Study Group. J Pediatr Hematol Oncol, 2007,29(1):27–31
Chen B, Wang YY, Shen Y, et al. Newly diagnosed acute lymphoblastic leukemia in China (I): abnormal genetic patterns in 1346 childhood and adult cases and their comparison with the reports from Western countries. Leukemia, 2012,26(7):1608–1616
Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N EngI J Med, 2015,373(16):1541–1552
Gmidène A, Sennana H, Elghezal H, et al. Cytogenetic analysis of 298 newly diagnosed cases of acute lymphoblastic leukaemia in Tunisia. Hematol Oncol, 2008,26(2):91–97
De Braekeleer E, Basinko A, Douet-Guilbert N, et al. Cytogenetics in pre-B and B-cell acute lymphoblastic leukemia: a study of 208 patients diagnosed between 1981 and 2008. Cancer Genet Cytogenet, 2010,200(1):8–15
Reismüller B, Steiner M, Pichler H, et al. High hyperdiploid acute lymphoblastic leukemia (ALL)-A 25-year population-based survey of the Austrian ALL-BFM (Berlin-Frankfurt-Münster) Study Group. Pediatr Blood Cancer, 2017,64(6)
De Lorenzo P, Moorman AV, Pieters R, et al. Cytogenetics and outcome of infants with acute lymphoblastic leukemia and absence of MLL rearrangements. Leukemia, 2014,28(2):428–30
Safavi S, Paulsson K. Near-haploid and low-hypodiploid acute lymphoblastic leukemia: two distinct subtypes with consistently poor prognosis. Blood, 2017,129(4): 420–423
Rachieru-Sourisseau P, Baranger L, Dastugue N, et al. DNA Index in childhood acute lymphoblastic leukaemia: a karyotypic method to validate the flow cytometric measurement. Int J Lab Hematol, 2010,32(3):288–298
Harrison CJ, Moorman AV, Broadfield ZJ, et al. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol, 2004,125(5):552–559
Attarbaschi A, Mann G, König M, et al. Incidence and relevance of secondary chromosome abnormalities in childhood TEL/AML1+ acute lymphoblastic leukemia: an interphase FISH analysis. Leukemia, 2004,18(10): 1611–1616
Kwon YJ, Lee JW, Kim MS, et al. Cytogenetic analysis in childhood acute lymphoblastic leukemia: experience at a single institution in Korea. Int J Hematol, 2009,89(2): 150–158
Bhandari P, Ahmad F, Dalvi R, et al. Cytogenetic Profile of De Novo B lineage Acute Lymphoblastic Leukemia: Determination of Frequency, Distribution Pattern and Identification of Rare and Novel Chromosomal Aberrations in Indian Patients. Asian Pac J Cancer Prev, 2015,16(16):7219–7229
Bhojwani D, Pei D, Sandlund JT, et al. ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia, 2012,26(2):265–270
Enshaei A, Schwab CJ, Konn ZJ, et al. Long-term follow-up of ETV6-RUNX1 ALL reveals that NCI risk, rather than secondary genetic abnormalities, is the key risk factor. Leukemia, 2013,27(11):2256–2259
Goud TM, Al Salmani KK, Al Harasi SM, Al Musalhi M, Wasifuddin SM, Rajab A. Importance of FISH combined with Morphology, Immunophenotype and Cytogenetic Analysis of Childhood/Adult Acute Lymphoblastic Leukemia in Omani Patients. Asian Pac J Cancer Prev, 2015,16(16):7343–7350
Prima V, Gore L, Caires A, et al. Cloning and functional characterization of MEF2D/DAZAP1 and DAZAP1/MEF2D fusion proteins created by a variant t(1;19) (q23;p13.3) in acute lymphoblastic leukemia. Leukemia, 2005,19(5):806–813
Gu Z, Churchman M, Roberts K, et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat Commun, 2016,7:13331
Suzuki K, Okuno Y, Kawashima N, et al. MEF2D-BCL9 Fusion Gene Is Associated With High-Risk Acute B-Cell Precursor Lymphoblastic Leukemia in Adolescents. J Clin Oncol, 2016,34(28):3451–3459
Hunger SP. Chromosomal translocations involving the E2A gene in acute lymphoblastic leukemia: clinical features and molecular pathogenesis. Blood, 1996,87(4): 1211–1224
Asai D, Imamura T, Yamashita Y, et al. Outcome of TCF3-PBX1 positive pediatric acute lymphoblastic leukemia patients in Japan: a collaborative study of Japan Association of Childhood Leukemia Study (JACLS) and Children’s Cancer and Leukemia Study Group (CCLSG). Cancer Med, 2014,3(3):623–631
Pang L, Liang Y, Pan J, et al. Clinical features and prognostic significance of TCF3-PBX1 fusion gene in Chinese children with acute lymphoblastic leukemia by using a modified ALL-BFM-95 protocol. Pediatr Hematol Oncol, 2015,32(3):173–181
Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia, 2009,23(8):1406–1409
Moorman AV. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica, 2016,101(4): 407–416
Beyermann B, Adams HP, Henze G. Philadelphia chromosome in relapsed childhood acute lymphoblastic leukemia: a matched-pair analysis. Berlin-Frankfurt-Münster Study Group. J Clinical Oncol, 1997,15(6): 2231–2237
Shen S, Chen X, Cai J, et al. Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA Oncol, 2020,16(3): 358–366
Mühlbacher V, Haferlach T, Kern W, et al. Array-based comparative genomic hybridization detects copy number variations with prognostic relevance in 80% of ALL with normal karyotype or failed chromosome analysis. Leukemia, 2016,30(2):318–324
Meyer C, Burmeister T, Gröger D, et al. The MLL recombinome of acute leukemias in 2017. Leukemia, 2018,32(2):273–284
Emerenciano M, Meyer C, Mansur MB, et al. The distribution of MLL breakpoints correlates with outcome in infant acute leukaemia. Br J Haematol, 2013,161(2): 224–236
Pieters R, De Lorenzo P, Ancliffe P, et al. Outcome of Infants Younger Than 1 Year With Acute Lymphoblastic Leukemia Treated With the Interfant-06 Protocol: Results From an International Phase III Randomized Study. J Clin Oncol, 2019,37(25):2246–2256
Cai J, Yu J, Zhu X, et al. Treatment abandonment in childhood acute lymphoblastic leukaemia in China: a retrospective cohort study of the Chinese Children’s Cancer Group. Arch Dis Child, 2019,104(6):522–529
Kim B, Kim E, Lee ST, et al. Detection of recurrent, rare, and novel gene fusions in patients with acute leukemia using next-generation sequencing approaches. Hematol Oncol, 2020,38(1):82–88
Simon P, Suciu S, Clappier E, et al. Different outcome of T cell acute lymphoblastic leukemia with translocation t(11;14) treated in two consecutive children leukemia group EORTC trials. Ann Hematol, 2016,95(1):93–103
Gachet S, El-Chaar T, Avran D, et al. Deletion 6q Drives T-cell Leukemia Progression by Ribosome Modulation. Cancer Discov, 2018,8(12):1614–1631
Mi JQ, Wang X, Yao Y, et al. Newly diagnosed acute lymphoblastic leukemia in China (II): prognosis related to genetic abnormalities in a series of 1091 cases. Leukemia, 2012,26(7):1507–1516
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Additional information
This study was supported by grants from the National Natural Science Foundation of China (No. 81670136), VIVA-China Children’s Cancer Foundation (Hong Kong), and Chinese Children’s Cancer Group-ALL subgroup.
Rights and permissions
About this article
Cite this article
Yin, Mm., Wu, Rc., Gao, J. et al. Cytogenetic Characteristics of Childhood Acute Lymphoblastic Leukemia: A Study of 1541 Chinese Patients Newly Diagnosed between 2001 and 2014. CURR MED SCI 42, 201–209 (2022). https://doi.org/10.1007/s11596-021-2477-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-021-2477-0