Summary
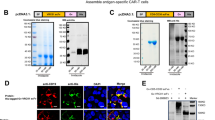
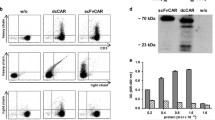
Selecting an ideal molecular format from diverse structures is a major challenge in developing a bispecific antibody (BsAb). To choose an ideal format of anti-CD3 × anti-transferrin receptor (TfR) bispecific antibodies for clinical application, we constructed TfR bispecific T-cell engager (BiTE) in two extensively applied formats, including single-chain tandem single-chain variable fragments (scFvs) and double-chain diabodies, and evaluated their functional characterizations in vitro. Results demonstrated that TfR-BiTE in both formats directed potent killing of TfR+ HepG2 cells. However, compared to two-chain diabodies, scFvs were more efficient in antigen binding and TfR+ target killing. Furthermore, different domain orders in scFvs would also be evaluated because single-TfR-CD3-His was preferable to single-CD3-TfR-His in immunotherapeutic strategies. Thus, the single-chain tandem TfR-CD3 format was favored for further investigation in cancer therapy.
Similar content being viewed by others
References
Ayyar BV, Arora S, O’Kennedy R. Coming-of-Age of Antibodies in Cancer Therapeutics. Trends Pharmacol Sci, 2016,37(12):1009–1028
Seimetz D, Lindhofer H, Bokemeyer C. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev, 2010,36(6):458–467
Batlevi CL, Matsuki E, Brentjens RJ, et al. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol, 2016,13(1):25–40
Velasquez MP, Bonifant CL. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood, 2018,131(1):30–38
Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res, 2009,69(12):4941–4944
Friberg G, Reese D. Blinatumomab (Blincyto): lessons learned from the bispecific t-cell engager (BiTE) in acute lymphocytic leukemia (ALL). Ann Oncol, 2017,28(8):2009–2012
Wu Z, Cheung NV. T cell engaging bispecific antibody (T-BsAb): From technology to therapeutics. Pharmacol Ther, 2018,182:161–175
Morrison SL. Two heads are better than one. Nat Biotechnol, 2007,25(11):1233–1234
Carter PJ, Lazar GA. Next generation antibody drugs: pursuit of the “high-hanging fruit”. Nat Rev Drug Discov, 2018,17(3):197–223
Wolf E, Hofmeister R, Kufer P, et al. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today, 2005,10(18):1237–1244
Fan G, Wang Z, Hao M, et al. Bispecific antibodies and their applications. J Hematol Oncol, 2015,8:130
Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today, 2015,20(7):838–847
Krishnamurthy A, Jimeno A. Bispecific antibodies for cancer therapy: A review. Pharmacol Ther, 2018,185:122–134
Holliger P, Prospero T, Winter G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci USA, 1993,90(14):6444–6448
Mack M, Riethmuller G, Kufer P. A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci USA, 1995,92(15):7021–7025
Garber K. Bispecific antibodies rise again. Nat Rev Drug Discov, 2014,13(11):799–801
Loffler A, Kufer P, Lutterbuse R, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood, 2000,95(6):2098–2103
Frankel SR, Baeuerle PA. Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol, 2013,17(3):385–392
Riethmuller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immun, 2012,12:12
Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs, 2017,9(2):182–212
Mau-Sorensen M, Dittrich C, Dienstmann R, et al. A phase I trial of intravenous catumaxomab: a bispecific monoclonal antibody targeting EpCAM and the T cell coreceptor CD3. Cancer Chemother Pharmacol, 2015,75(5):1065–1073
Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol, 2015,67(2 Pt A):95–106
Kipriyanov SM. Generation and characterization of bispecific tandem diabodies for tumor therapy. Methods Mol Biol, 2003,207:323–333
Schmiedl A, Breitling F, Dubel S. Expression of a bispecific dsFv-dsFv’ antibody fragment in Escherichia coli. Protein Eng, 2000,13(10):725–734
Mabry R, Lewis KE, Moore M, et al. Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng Des Sel, 2010,23(3):115–127
Huang BC, Foote LJ, Lankford TK, et al. A diabody that dissociates to monomer forms at low concentration: effects on binding activity and tumor targeting. Biochem Biophys Res Commun, 2005,327(4):999–1005
Compte M, Alvarez-Cienfuegos A, Nunez-Prado N, et al. Functional comparison of single-chain and two-chain anti-CD3-based bispecific antibodies in gene immunotherapy applications. Oncoimmunology, 2014,3(5):e28810
Jost C, Pluckthun A. Engineered proteins with desired specificity: DARPins, other alternative scaffolds and bispecific IgGs. Curr Opin Struct Biol, 2014,27:102–112
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest Statement
The authors have declared that no conflict of interest exists.
This research was supported by grants from the National Natural Science Foundation of China (No. 31570937 and No. 81871391), Natural Science Foundation of Hubei Province of China (No. 2017CFB707), the Fundamental Research Funds for the Central Universities of China (No. HUST: 2018KFYYXJJ086) and Graduates’ Innovation Foundation of Huazhong University of Science and Technology (No. 5003510001).
Rights and permissions
About this article
Cite this article
Fu, Mp., Guo, Zl., Tang, Hl. et al. Selection for Anti-transferrin Receptor Bispecific T-cell Engager in Different Molecular Formats. CURR MED SCI 40, 28–34 (2020). https://doi.org/10.1007/s11596-020-2143-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-020-2143-y