Summary
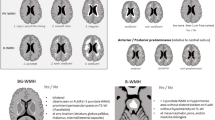
Brain magnetic resonance imaging (MRI) of the elderly often reveals white matter changes (WMCs) with substantial variability across individuals. Our study was designed to explore MRI features and site-specific factors of ischemic WMCs. Clinical data of consecutive patients diagnosed with ischemic cerebral vascular disease who had undergone brain MRI were collected and analyzed. Multi-logistic regression analysis comparing patients with mild versus severe WMCs was performed to detect independent associations. Analyses of variance (ANOVAs) were used to detect regionally specific differences in lesions. We found that lesion distribution differed significantly across five cerebral areas, with lesions being predominant in the frontal lobe and parieto-occipital area. To explore WMCs risk factors, after adjusting for gender, diabetes mellitus, and hypertension, only age (P<0.01), creatinine (P=0.01), alkaline phosphatase (ALP) (P=0.01) and low-density lipoprotein cholesterol (LDL-C) (P=0.03) were found to be independently associated with severe WMCs. Age (P<0.001) was strongly associated with WMCs in the frontal lobe while hypertension was independently related to lesions in the basal ganglia (P=0.048) or infratentorial area (P=0.016). In conclusion, MRI of WMCs showed that ischemic WMCs occurred mostly in the frontal lobe and parieto-occipital area. The infratentorial area was least affected by WMCs. Typically, age-related WMCs were observed in the frontal lobes, while hypertension-related WMCs tended to occur in the basal ganglia and infratentorial area.
Similar content being viewed by others
References
O’Sullivan M. Leukoaraiosis. Pract Neurol, 2008,8(1):26–38
Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and metaanalysis. BMJ, 2010,341:c3666
Ginsberg MD, Hedley-Whyte ET, Richardson EP. Hypoxic-ischemic leukoencephalopathy in man. Arch Neurol, 1976,33(1):5–14
Inzitari D, Mascalchi M, Giordano GP, et al. Histopathological correlates of leuko-araiosis in patients with ischemic stroke. Eur Neurol, 1989,29(Suppl 2):23–26
Wardlaw JM, Sandercock PA, Dennis MS, et al. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia. Stroke, 2003,34(3):806–812
Hassan A, Hunt BJ, O’Sullivan M, et al. Markers of endothelial dysfunction in lacunar infarction and ischaemic leukoaraiosis. Brain, 2003,126(Pt 2):424–432
Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology, 1994,44(7):1246–1252
Havlik RJ, Foley DJ, Sayer B, et al. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke, 2002,33(1):26–30
de Leeuw FE, Richard F, de Groot JC, et al. Interaction between hypertension, apoE, and cerebral white matter lesions. Stroke, 2004,35(5):1057–1060
Anan F, Masaki T, Kikuchi H, et al. Association between plasma high-sensitivity C-reactive protein and insulin resistance and white matter lesions in Japanese type 2 diabetic patients. Diabetes Res Clin Pract, 2010,87(2):233–239
The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. WHO MONICA Project Principal Investigators. J Clin Epidemiol, 1988,41(2):105-114
Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ, 1976,54(5):541–553
Wahlund LO, Barkhof F, Fazekas F, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke, 2001,32(6):1318–1322
Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60-to 64-year-old individuals. Neuroimage, 2004,22(1):144–154
Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS). Stroke, 2007,38(12):3121–3126
Shlipak MG, Fried LF, Crump C, et al. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation, 2003,107(1):87–92
Stuveling EM, Hillege HL, Bakker SJ, et al. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int, 2003,63(2):654–661
Longstreth WT, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke, 1996,27(8):1274–1282
de Leeuw FE, de Groot JC, Oudkerk M, et al. A follow-up study of blood pressure and cerebral white matter lesions. Ann Neurol, 1999,46(6):827–833
Ryu WS, Lee SH, Kim CK, et al. High serum ALP in relation to cerebral small vessel disease. Atherosclerosis, 2014,232(2):313–318
Harmey D, Hessle L, Narisawa S, et al. Concerted regulation of inorganic pyrophosphate and osteopontin by akp2, enpp1, and ank: an integrated model of the pathogenesis of mineralization disorders. Am J Pathol, 2004,164(4):1199–1209
Shroff RC, McNair R, Figg N, et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation, 2008,118(17):1748–1757
Vidal JS, Sigurdsson S, Jonsdottir MK, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke, 2010,41(5):891–697
Bos D, Ikram MA, Elias-Smale SE, et al. Calcification in major vessel beds relates to vascular brain disease. Arterioscler Thromb Vasc Biol, 2011,31(10):2331–2337
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from Scientific Research Project of Health and Family Planning of Hubei Province (No. WJ2015MB056), Natural Science Foundation of Hubei Province (No. 2015CFB572), and Clinical Research Physician Program of Tongji Medical College, HUST.
Rights and permissions
About this article
Cite this article
Zhang, Yp., Liu, N., Liu, Ky. et al. MRI Features and Site-specific Factors of Ischemic Changes in White Matter: A Retrospective Study. CURR MED SCI 38, 318–323 (2018). https://doi.org/10.1007/s11596-018-1881-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-018-1881-6