Summary
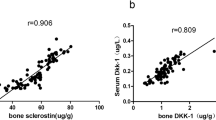
Wnt signaling plays an important role in the bone development and remodeling. The Wnt antagonist Dkk-1 is a potent inhibitor of bone formation. The aims of this study were firstly to compare the serum Dkk-1 levels in postmenopausal osteoporosis patients with age-matched healthy controls, and secondly, to assess the possible relationship between Dkk-1 and β-catenin, sclerostin, or bone turnover markers [CTX, PINP, N-MID-OT and 25(OH)D] in the setting of postmenopausal osteoporosis. A total of 350 patients with postmenopausal osteoporosis and 150 age-matched healthy controls were enrolled, and the serum levels of Dkk-1, β-catenin, sclerostin, OPG, and RANKL were detected by ELISA, and bone turnover markers [CTX, PINP, N-MID-OT and 25(OH)D] were measured by Roche electrochemiluminescence system in two groups. Serum Dkk-1 levels were significantly higher in postmenopausal osteoporosis group than in control group (P<0.001). Univariate analyses revealed that serum Dkk-1 levels were weakly negatively correlated to β-catenin (r=−0.161, P=0.003) and OPG (r=−0.106, P=0.047), while multiple regression analysis showed a negative correlation between serum Dkk-1 levels with β-catenin (β=−0.165, P=0.009) and BMD (β=−0.139, P=0.027), and a positive correlation between serum Dkk-1 levels and CTX (β=0.122, P=0.040) in postmenopausal osteoporosis group. No similar correlations ware observed in control group. The results provided evidence for the role of Dkk-1 in bone metabolism and demonstrated the link of Dkk-1 and Wnt/β-catenin in some ways.
Similar content being viewed by others
References
Sambrook P, Cooper C. Osteoporosis. Lancet, 2006,367:2010–2018
Gifre L, Ruiz-Gaspà S, Monegal A, et al. Effect of glucocorticoid treatment on Wnt signalling antagonists (sclerostin and Dkk-1) and their relationship with bone turnover. Bone, 2013,57(1):272–276
Chamorro MN, Schwartz DR, Vonica A, et al. FGF-20 and DKK1 are transcriptional targets of beta-catenin and FGF-20 is implicated in cancer and development. EMBO J, 2005,24(1):73–84
Voorzaner-Rousselot N, Goehrig D, Facon T, et al. Platelet is a major contributor to circulating levels of Dickkopf-1: clinical implications in patients with multiple myeloma. Bri J Haematol, 2009,145(2):264–266
Pinzone JJ, Hall BM, Thudi NK, et al. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood, 2009,113(3):517–525
Morvan F, Boulukos K, Clément-Lacroix P, et al. Deletion of a single allele of the Dkk1 gene leads to an increase in bone formation and bone mass. J Bone Mineral Res, 2006,21(6):934–945
Li J, Sarosi I, Cattley RC, et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone, 2006,39(4):754–766
Wang FS, Ko JY, Yeh DW, et al. Modulation of dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology, 2008,149(4):1793–1801
Wang FS, Ko JY, Lin CL, et al. Knocking down dickkopf-1 alleviates estrogen deficiency induction of bone loss. A histomorphological study in ovariectomized rats. Bone, 2007,40(2):485–492
Terpos E, Dimopoulos MA, Sezer O. The effect of novel anti-myeloma agents on bone metabolism of patients with multiple myeloma. Leukemia, 2007,21(9):1875–1884
Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Inv, 2006,116(5):1202–1209
Rawadi G, Vayssière B, Dunn F, et al. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Mineral Res, 2003,18(10):1842–1853
Suzuki A, Ozono K, Kubota T, et al. PTH/cAMP/PKA signaling facilitates canonical Wnt signaling via inactivation of glycogen synthase kinase-3beta in osteoblastic Saos-2 cells. J Cell Biochem, 2008,104(1):304–317
Xu XJ, Shen L, Yang YP, et al. Serum β-catenin levels associated with the ratio of RANKL/OPG in patients with postmenopausal osteoporosis. Int J Endocrinol, 2013,2013:534 352
Albers J, Keller J, Baranowsky A, et al. Canonical Wnt signaling inhibits o-steoclastogenesis independent of osteoprotegerin. J Cell Biol, 2013,200(4):537–549
Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell, 2012,149(6):1192–1205
Essers MA, de Vries-Smits LM, Barker N, et al. Functional interaction between beta-catenin and FOXO in oxdative stress signaling. Science, 2005,308(5725):1181–1184
Anastasilakis AD, Polyzos SA, Toulis KA. Role of wingless tail signaling pathway in osteoporosis: an update of current knowledge. Curr Opin Endocrinol Diabetes Obes, 2011,18(6):383–388
Drake MT, Srinivasan B, Mödder UI, et al. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab, 2010,95(11):5056–5062
Xu XJ, Shen L, Yang YP, et al. Serum sclerostin levels associated with lumbar spine bone mineral density and bone turnover markers in patients with postmenopausal osteoporosis. Chin Med J, 2013,126(13):2480–2484
Kyvernitakis I, Rachner TD, Urbschat A, et al. Effect of aromatase inhibition on serum levels of sclerostin and dickkopf-1, bone turnover markers and bone mineral density in women with breast cancer. J Cancer Res Clin Oncol, 2014,140(10):1671–1680
Voorzanger-Rousselot N, Journe F, Doriath V, et al. Assessment of circulating dickkopf-1 with a new two-site immunoassay in healthy subjects and women with breast cancer and bone metastases. Calcifi Tissue Int, 2009,84(5):348–354
Anastasilakis AD, Polyzos SA, Avramidis A, et al. The effect of teriparatide on serum Dickkopf-1 levels in postmenopausal women with established osteoporosis. Clin Endocrinol (Oxf), 2010,72(6):752–757
Bafico A, Liu GZ, Yaniv A, et al. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol, 2001,3(7):683–686
Davidson G, Mao BY, Barrantes ID, et al. Kremen proteins interact with Dickkopf1 to regulate anteroposterior CNS patterning. Development, 2002,129(24):5587–5596
Gregory CA, Singh H, Perry AS, et al. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem, 2003,278(30):28 067–28 078
Chu EY, Hens J, Andl T, et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development, 2004,131(19):4819–4829
Kuhnert F, Davis CR, Wang HT, et al. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc atl Acad Sci USA, 2004,101(1):266–271
Voskaridou E, Christoulas D, Xirakia C, et al. Serum Dickkopf-1 is increased and correlates with reduced bone mineral density in patients with thalassemia-induced osteoporosis. Reduction post-zoledronic acid administration. Haematologica, 2009,94(5):725–728
Aguilera O, Peña C, García JM, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihyroxy vitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis, 2007,28(9):1877–1884
Glass DA 2nd, Karsenty G. Canonical Wnt signalin-in osteoblasts is required for osteoclast differentiation. Ann New York Acad Sci, 2006,1068:117–130
Diarra D, Stolina M, Polzer K, et al. Dickkopf-1 is a master regulator of joint remodeling. Nature Med, 2007,13(2):156–163
Bellido T, Ali AA, Gubrij I, et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology, 2005,146(11):4577–4583
Guo J, Liu M, Yang D, et al. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab, 2010,11(2):161–171
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors contributed equally to this work.
This project was supported by grants from National Natural Science Foundation of China (No. 81473492 and No. 81273907), and Health Department of Hubei Province, China (No. 2012Z-Z01).
Rights and permissions
About this article
Cite this article
Tian, J., Xu, Xj., Shen, L. et al. Association of serum Dkk-1 levels with β-catenin in patients with postmenopausal osteoporosis. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 35, 212–218 (2015). https://doi.org/10.1007/s11596-015-1413-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-015-1413-6