Abstract
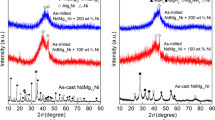
The PrMg12-type composite alloy of PrMg11Ni + x wt% Ni (x = 100, 200) with an amorphous and nanocrystalline microstructure were synthesized through the mechanical milling. Effects of milling duration and Ni content on the microstructures and electrochemical hydrogen storage performances of the ball-milled alloys were methodically studied. The ball-milled alloys obtain the optimum discharge capacities at the first cycle. Increasing Ni content dramatically enhances the electrochemical property of alloys. Milling time varying may obviously impact the electrochemical performance of these alloys. The discharge capacities show a significant upward trend with milling duration prolonging, but milling for a longer time more than 40 h induces a slight decrease in the discharge capacity of the x = 200 alloy. As milling duration increases, the cycle stability clearly lowers, while it first declines and then augments under the same condition for the x = 200 alloy. The high-rate discharge abilities of the ball-milled alloys show the optimum values with milling time varying.
Similar content being viewed by others
References
Mori D, Hirose K. Recent Challenges of Hydrogen Storage Technologies for Fuel Cell Vehicles[J]. Int. J. Hydrogen Energy, 2009, 34(10): 4 569–4 574
Lan R, Irine JTS, Tao S. Ammonia and Related Chemicals as Potential Indirect Hydrogen Storage Materials[J]. Int. J. Hydrogen Energy, 2012, 37(2): 1 482–1 494
Luo Q, Li J, Li B, et al. Kinetics in Mg-based Hydrogen Storage Materials: Enhancement and Mechanism[J]. J. Magnesium Alloys, 2019, 7(1): 58–71
Marques F, Pinto HC, Figueroa SJA, et al. Mg-containing Multi-principal Element Alloys for Hydrogen Storage: A Study of the MgTiNbCr0.5 Mn0.5Ni0.5 and Mg0.68TiNbNi0.55 Compositions[J]. Int. J. Hydrogen Energy, 2020, 45(38): 19 539–19 552
Song MY, Choi E, Kwak YJ. Synthesis of a Mg-based Alloy with a Hydrogen-storage Capacity of Over 7 wt% by Adding a Polymer CMC via Transformation-involving Milling[J]. Mater. Res. Bull., 2018, 108: 23–31
Liu T, Wang CX, Wu Y. Mg-based Nanocomposites with Improved Hydrogen Storage Performances[J]. Int. J. Hydrogen Energy, 2014, 39(26): 14 262–14 274
Klebanoff LE, Keller JO. 5 Years of Hydrogen Storage Research in the U.S. DOE Metal Hydride Center of Excellence (MHCOE)[J]. Int. J. Hydrogen Energy, 2013, 38(11): 4 533–4 576
Makridis SS, Gkanas EI, Panagakos G, et al. Polymer-stable Magnesium Nanocomposites Prepared by Laser Ablation for Efficient Hydrogen Storage[J]. Int. J. Hydrogen Energy, 2013, 38(26): 11 530–11 535
Eftekhari A, Fang B. Electrochemical Hydrogen Storage: Opportunities for Fuel Storage, Batteries, Fuel Cells, and Supercapacitors[J]. Int. J. Hydrogen Energy, 2017, 42(40): 25 143–25 165
Wang Y, Wang X, Li CM. Electrochemical Hydrogen Storage of Ball-milled MmMg12 Alloy-Ni Composites[J]. Int. J. Hydrogen Energy, 2010, 35(8): 3 550–3 554
Bu W, Zhang W, Gao J, et al. Improved Hydrogen Storage Kinetics of Nanocrystalline and Amorphous Pr-Mg-Ni-based PrMg12-type Alloys Synthesized by Mechanical Milling[J]. Int. J. Hydrogen Energy, 2017, 42(29): 18 452–18 464
Luo S, Li S, Liu Y, et al. Synergistically Tuned Hydrogen Storage Thermodynamics and Kinetics of Mg-Al Alloys by Cu Formed in Situ Mechanochemically[J]. J. Alloys Compd., 2019, 806: 370–377
Yong H, Guo S, Yuan Z, et al. Improved Hydrogen Storage Kinetics and Thermodynamics of RE-Mg-based Alloy by Co-doping Ce-Y[J]. Int. J. Hydrogen Energy, 2019, 44(31): 16 765–16 776
Lim KL, Liu Y, Zhang QA, et al. Cycle Stability of La-Mg-Ni Based Hydrogen Storage Alloys in a Gas-solid Reaction[J]. Int. J. Hydrogen Energy, 2017, 42(37): 23 737–23 745
Zhang L, Du W, Han S, et al. Study on Solid Solubility of Mg in Pr3−x MgxNi9 and Electrochemical Properties of PuNi3-type Single-phase RE-Mg-Ni (RE = La, Pr, Nd) Hydrogen Storage Alloys[J]. Electrochim. Acta, 2015, 173: 200–208
Xu C, Lin HJ, Wang Y, et al. Catalytic Effect of in Situ Formed Nano-Mg2Ni and Mg2Cu on the Hydrogen Storage Properties of Mg-Y Hydride Composites[J]. J. Alloys Compd., 2019, 782: 242–250
Li B, Li J, Zhao H, et al. Mg-based Metastable Nano Alloys for Hydrogen Storage[J]. Int. J. Hydrogen Energy, 2019, 44(12): 6 007–6 018
Song MY, Choi E, Kwak YJ. Increase in the Dehydrogenation Rates and Hydrogen-storage Capacity of Mg-graphene Composites by Adding Nickel via Reactive Ball Milling[J]. Mater. Res. Bull., 2020, 130: 110–938
Lv W, Wu Y. Effect of Melt Spinning on the Structural and Low Temperature Electrochemical Characteristics of La-Mg-Ni Based La0.65Ce0.1Mg0.25Ni3Co0.5 Hydrogen Storage Alloy[J]. J. Alloys Compd., 2019, 789: 547–557
Li Y, Liu Z, Zhang G, et al. Single Phase A2B7-type La-Mg-Ni Alloy with Improved Electrochemical Properties Prepared by Melt-spinning and Annealing[J]. J. Rare Earths, 2019, 37(12): 1 305–1 311
Huang J, Wang H, Ouyang L, et al. Reducing the Electrochemical Capacity Decay of Milled Mg-Ni Alloys: The Role of Stabilizing Amorphous Phase by Ti-substitution[J]. J. Power Sources, 2019, 438: 226–984
Hu F, Luo L, Cai Y, et al. Investigation of Microstructure and Electrochemical Hydrogen Storage Thermodynamic and Kinetic Properties of Ball-milled CeMg12-type Composite Materials[J]. Mater. Des., 2019, 182: 108–034
Abdellaoui M, Mokbli S, Cuevas F, et al. Structural and Electrochemical Properties of Amorphous Rich MgxNi100−x Nanomaterial Obtained by Mechanical Alloying[J]. J. Alloys Compd., 2003, 356–357: 557–565
Lai WH, Yu CZ. Research Survey of Improving Discharge Voltage Platform for Ni-MH Battery[J]. Battery Bimon., 1996, 26(4): 189–191
Spassov T, Lyubenova L, Köster U, et al. Mg-Ni-RE Nanocrystalline Alloys for Hydrogen Storage[J]. Mater. Sci. Eng. A, 2004, 375–377: 794–799
Zhao XY, Ding Y, Ma LQ, et al. Electrochemical Properties of MmNi3.8 Co0.75Mn0.4Al0.2 Hydrogen Storage Alloy Modified with Nanocrystalline Nickel[J]. Int. J. Hydrogen Energy, 2008, 33(22): 6 727–6 733
Wang Y, Lu ZW, Gao XP, et al. Electrochemical Properties of the Ball-milled LaMg10Ni2−xAlx Alloys with Ni Powders (x = 0, 0.5, 1 and 1.5) [J]. J. Alloys Compd., 2005, 389(1-2): 290–295
Simičić MV, Zdujić M, Dimitrijević R, et al. Hydrogen Absorption and Electrochemical Properties of Mg2Ni-type Alloys Synthesized by Mechanical Alloying[J]. J. Power Sources, 2006, 158(1): 730–734
Zhu D, Zhang J, Zhu Y, et al. Electrochemical Hydrogen Storage Properties of Mg100−xNix Produced by Hydriding Combustion Synthesis and Mechanical Milling[J]. Prog. Nat. Sci. -Mater., 2017, 27(1): 144–148
Zheng G, Popov BN, White RE. Electrochemical Determination of the Diffusion Coefficient of Hydrogen Through an LaNi4.25Al0.75 Electrode in Alkaline Aqueous Solution[J]. J. Electrochem. Soc., 1995, 142(8): 2 695–2 698
Kuriyama N, Sakai T, Miyamura H, et al. Electrochemical Impedance and Deterioration Behavior of Metal Hydride Electrodes[J]. J. Alloys Compd., 1993, 202(1-2): 183–197
Zhang YH, Yuan ZM, Zhai TT, et al. Electrochemical Hydrogen Storage Behaviors of the Nanocrystalline and Amorphous Nd-Cu-added Mg2Ni-type Alloy Electrodes Applied to Ni-MH Battery[J]. J. Solid State Electrochem., 2015, 19(8): 2 343–2 351
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by National Natural Science Foundation of China (Nos. 51871125, 51901105 and 51761032) and Inner Mongolia Natural Science Foundation (No.2019BS05005)
Rights and permissions
About this article
Cite this article
Hou, Z., Yuan, Z., Feng, D. et al. Electrochemical Hydrogen Storage Performance of the Nanocrystalline and Amorphous Pr-Mg-Ni-based Alloys Synthesized by Mechanical Milling. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 36, 116–126 (2021). https://doi.org/10.1007/s11595-021-2384-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-021-2384-z