Abstract
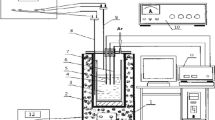
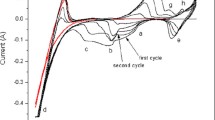
Vanadium trioxide (V2O3) was directly prepared by NaVO3 electrolysis in NaCl molten salts. Electrolysis products were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS). The existing state and electrochemical behavior of NaVO3 were also studied. The results indicated that V2O3 can be obtained from NaVO3. VC and C were also formed at high cell voltage, high temperature, and long electrolysis time. During electrolysis, NaVO3 was dissociated to Na+ and VO3 − in NaCl molten salt. NaVO3 was initially electro- reduced to V2O3 on cathode and Na2O was released simultaneously. Na2CO3 was formed due to the reaction between Na2O and CO2. The production of C was ascribed to the electro-reduction of CO3 2−. VC was produced due to the reaction between C and V2O3.
Similar content being viewed by others
References
Zhang Y F, Zhang F F, Yu L, et al. Synthesis and Characterization of Belt-like VO2(B)@carbon and V2O3@carbon Core-shell Structured Composites[J]. Colloid Surface A, 2012, 396(20): 144–152
Zheng C M, Zhang X M, He S, et al. Preparation and Characterization of Spherical V2O3 Nano Powder[J]. J. Solid State Chem., 2003, 170: 221–226
Chen W, Xu Q, Cui W Q. Effects of Quenching Treatment on the Structure and Electrical Property in Doped V2O3 System[J]. J. Mater. Sci., 1997, 32(4): 1049–1053
Kong F Y, Li M, Li D B, et al. Synthesis and Characterization of V2O3 Nanocrystals by Plasma Hydrogen Reduction[J]. J. Crystal Growth, 2012, 346: 22–26
Kittaka S, Sasaki S, Morimoto T. Spherical Particles and Their Surface Properties[J]. J. Mater. Sci., 1987, 22(2): 557–564
Moskalyk R R, Alfantazi A M. Processing of Vanadium: a Review[J]. Miner. Eng., 2003, 16(9): 793–805
Li X S, Xie B, Wang G E, et al. Oxidation Process of Low-grade Vanadium Slag in Presence of Na2CO3[J]. Trans. Nonferrous Met. Soc. China, 2011, 21(8): 1860–1867
Aarabi-Karasgani M, Rashchi F, Mostoufi N, et al. Leaching of Vanadium from LD Converter Slag Using Sulfuric Acid[J]. Hydrometallurgy, 2010, 102(1-2): 14–21
Shirinov E G, Gasanly Z G, Ganbarov D M. Reduction of Vanadium from Alkaline Solutions[J]. Russ. J. Appl. Chem., 2009, 82(7): 1230–1233
Lingane J J. Polarographic Characteristics of Vanadium in its Various Oxidation States[J]. J. Am. Chem. Soc., 1945, 67(2): 182–188
Schmidt R W, Reilly C N. Concerning the Effect of Surface-active Substances on Polarographic Current[J]. J. Am. Chem. Soc., 1958, 80(9): 2087–2094
Cakir S, Bicer E. Voltammetric and Spectroscopic Studies of Vanadium( V)-Nicotinamide Interactions at Physiological pH[J]. Turk. J. Chem., 2007, 31: 223–227
Abouatallah R, Kirk D, Thorpe S, et al. Reactivation of Nickel Cathodes by Dissolved Vanadium Species during Hydrogen Evolution in Alkaline Media[J]. Electrochim. Acta, 2001, 47(4): 613–621
Abouatallah R M, Kirk D W, Thorpe S J. Characterization of Vanadium Deposit Formation at a Hydrogen Evolving Electrode in Alkaline Media[J]. J. Electrochem. Soc., 2001, 148(9): E357–E363
Birke R L, Santa Cruz T D. Electroreduction of Vanadium(V) in the Presence and Absence of Chlorite Ion[J]. J. Electrochem. Soc., 1973, 120(3): 366–373
Liu B, Zheng S L, Wang S N, et al. The Redox Behavior of Vanadium in Alkaline Solutions by Cyclic Voltammetry Method[J]. Electrochim. Acta, 2012, 76: 262–269
Gasviani N A, Khutsishvili M S, Abazadze L M. Electrochemical Reduction of Sodium Metavanadate in an Equimolar KCl–NaCl Melt[J]. Russ. J. Electrochem., 2006, 42(9): 931–937
Solheim A. Liquidus Temperature Depression in Cryolitic Melts[J]. Metall. Mater. Trans. B, 2012, 43B: 995–1000
Solheim A, Rolseth S, Skybakmoen E, et al. Liquidus Temperatures for Primary Crystallization of Cryolite in Molten Salt Systems of Interest for Aluminum Electrolysis[J]. Metall. Mater. Trans. B, 1996, 27B: 739–744
Hur J M, Cha J S, Choi E Y. Can carbon Be an Anode for Electrochemical Reduction in a LiCl-Li2O Molten Salt[J]. ECS Electrochem. Lett., 2014, 3(10): E5–E7
Jiao S Q, Fray D J. Development of an Inert Anode for Electrowinning in Calcium Chloride–Calcium Oxide Melts[J]. Metall. Mater. Trans. B, 2010, 41(1): 74–79
Kruesi W H, Fray D J. Fundamental Study of the Anodic and Cathodic Reactions during the Electrolysis of a Lithium Carbonate-Lithium Chloride Melt Using a Carbon Anode[J]. J. Appl. Electrochem., 1994, 24(11): 1102–1108
Ijije H V, Lawrence R C, Chen G Z. Carbon Electrodeposition in Molten Salts: Electrode Reactions and Applications[J]. RSC Adv., 2014, 67: 35808–35817
Li L X, Shi Z N, Gao B L, et al. Electrochemical Behavior of Carbonate Ion in the LiF–NaF–Li2CO3 System[J]. Electrochemistry, 2014, 82(12): 1072–1077
Kaplan B, Groult H, Barhoun A, et al. Synthesis and Structural Characterization of Carbon Powder by Electrolytic Reduction of Molten Li-2CO3-Na2CO3-K2CO3[J]. J. Electrochem. Soc., 2002, 149(5): D72–D78
Kawamura H, Ito Y. Electrodeposition of Cohesive Carbon Films on Aluminum in a LiCl–KCl–K2CO3 Melt[J]. J. Appl. Electrochem., 2000, 30: 571–574
Keer H V, Dickerson D L, Kuwamoto H, et al. Heat Capacity of Pure and Doped V2O3 Single Crystals[J]. J. Solid State Chem., 1976, 19: 95–102
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is supported by the National Basic Research Program of China (973 Program) (No.2013CB632606), National Natural Science Foundation of China (Nos.51474200, 51422405) and Youth Innovation Promotion Association, CAS (No.2015036)
Rights and permissions
About this article
Cite this article
Weng, W., Wang, M., Gong, X. et al. Electrochemical preparation of V2O3 from NaVO3 and its reduction mechanism. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 32, 1019–1024 (2017). https://doi.org/10.1007/s11595-017-1705-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-017-1705-8