Abstract
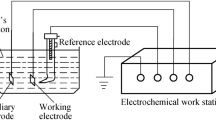
The corrosion mechanism of Zn-Cu-Ti alloy added with La in 3% NaOH solution was investigated by electrochemical testing and SEM observation. Polarization curves manifested that the overall corrosion kinetics of alloys are under anodic control. The anodic passivation of the Zn-Cu-Ti alloy is remarkably improved by the addition of La. Because La can effectively improve the hydrogen evolution/oxygen reduction over-potential of alloy elements, and the rare earth oxide film plays an important role in insulation that can strengthen the dielectric properties of Zn-Cu-Ti alloy, the corrosion resistance of Zn-Cu-Ti alloy is made significantly better by adding a trace amount of La. The improvement of corrosion resistance is not positively correlated with the adding amount of La to alloy. The Zn-Cu-Ti-0.5La alloy displays the best corrosion resistance behavior. The corrosion form of the alloys mainly belongs to a selective corrosion and the main solid corrosion products are Zn(OH)2 and ZnO.
Similar content being viewed by others
References
Caplat C, Mottin E, Lebel JM, et al. Impact of a Sacrificial Anode as Assessed by Zinc Accumulation in Different Organs of the Oyster Crassostrea gigas: Results from Long-and Short-Term Laboratory Tests[J]. Archives of Environmental Contamination & Toxicology, 2012, 62(4): 638–649
Mao A, Barillier D, and Guida M. Comparative Toxicities of Aluminum and Zinc from Sacrificial Anodes or from Sulfate salt in Sea Urchin Embryos and Sperm[J]. Ecotoxicology & Environmental Safety, 2010, 73(6): 1138–1143
Jin A J, Jin CK. Effect of Cathodic Protection Systems with Zn-Mesh Sacrificial Anodes on Reinforced Concrete Structures in an Accelerated Marine Environment[J]. Advanced Materials Research, 2013, 800: 365–374
Ferguson JB, Schultz BF, Rohatgi PK. Zinc Alloy ZA-8/shape Memory Alloy Self-healing Metal Matrix Composite[J]. Materials Science & Engineering A, 2014, 620: 85–88
Alhamidi A, Edalati K, Horita Z, et al. Softening by Severe Plastic Deformation and Hardening by Annealing of Aluminum-Zinc Alloy: Significance of Elemental and Spinodal Decompositions[J]. Materials Science & Engineering A, 2014, 610(29): 17–27
Zhang F, Wan JG. Effect of Cooling Mode on Properties of a Cast Zinc Alloy[J]. Advanced Materials Research, 2013, 690-693: 66–69
Zühlke T, Alkorta J, García-Rosales C, et al. Structure and Texture of Twin Roll Cast Strips of Zn-Cu-Ti Zinc Alloy[J]. Materials Sciences & Technology, 2014, 30(1): 91–95
Nasch T, Jeitschko W, Rodewald, UC. Ternary Rare Earth Transition Metal Zinc Compounds RT2Zn20 with T = Fe, Ru, Co, Rh, and Ni[J]. Zeitschrift Für Naturforschung B, 2014, 52(9): 1023–1030
LV YL, SHAO GJ, ZHAO BL, et al. Influence of La-dopant on the Material Characteristics and Supercapacitive Performance of MnO2 Electrodes[J]. Journal of Wuhan University of Technology, 2011 (01): 33–37
Xu CX, Wang X, Zhang JS, et al. Effect of Nd and Yb on the Microstructure and Mechanical Properties of Mg-Zn-Zr Alloy[J]. Rare Metal Materials & engineering, 2014, 43: 1809–1814
El-Sayed AR, Mohran HS, El-Lateef HMA. Effect of Minor Nickel Alloying with Zinc on the Electrochemical and Corrosion Behavior of Zinc in Alkaline Solution[J]. Journal of Power Sources, 2010, 195(19): 6924–6936
Arenas MA, Conde A, Damborenea JJD. Cerium: a Suitable Green Corrosion Inhibitor for Tinplate[J]. Corrosion Science, 2002, 44(3): 511–520
Palanivel V, Huang Y, Ooij WJV. Effects of Addition of Corrosion Inhibitors to Silane Films on the Performance of AA2024-T3 in a 0. 5M NaCl Solution[J]. Progress in Organic Coatings, 2005, 53(2): 153–168
Yuan TC, Zhou KC, Rui-Di LI. Effect of Additives Pr2O3 and Er2O3 on Properties of Electrodeposited Nickel Sulphur Alloy Electrode[J]. Chinese Journal of Nonferrous Metals, 2008 (5): 862–866(in Chinese)
Guessoum K, Veys-Renaux D, Rocca E, et al. Corrosion Behaviour of Zinc-Cerium Alloys: Role of Intermetallic Phases[J]. Corrosion Science, 2011, 53(5): 1639–1645
Rosalbino F, Angelini E, Macciò D, et al. Application of EIS to Assess the Effect of Rare Earths Small Addition on the Corrosion Behaviour of Zn-5% Al (Galfan) Alloy in Neutral Aerated Sodium Chloride Solution[J]. Electrochimica Acta, 2009, 54(4): 1204–1209
Amin MA. Passivity and Passivity Breakdown of a Zinc Electrode in Aerated Neutral Sodium Nitrate Solutions[J]. Electrochimica Acta, 2005, 50(6): 1265–1274
Li G, Wang H, Zhao Y, et al. Effect Mechanism of Yttrium on Melting and Solidification of 7055 Aluminum Alloy[J]. Rare Metal Materials & Engineering, 2010, 39(1): 80–84
Zhang J, Tang D, Zhang H, et al. Effect and Application of Rare Earth Element in Magnesium Alloys[J]. Chinese Journal of Rare Metals, 2008, 32(5): 659–667
Duan RB, Bai PK, Yang J, et al. Influence of Rare Earth Modification and Homogenization on the Microstructure and Mechanical Properties of Recycled Can 3004 Aluminum[J]. Journal of Wuhan University of Technology -Materials Science Edition, 2014, 29(2): 264–268
Veys-Renaux D, Guessoum K, Rocca E, et al. New Zinc-rare Earth alloys: Influence of Intermetallic Compounds on the Corrosion Resistance[J]. Corrosion Science, 2013, 77(1): 342–349
Guerrero R, Farias MH, Cota-Araiza L. Increase in Corrosion Resistance of Zn-22Al-2Cu Alloy by Depositing an Y2O3 Film Studied by Auger Electron Spectroscopy[J]. Applied Surface Science, 2002, 185(s 3-4): 248–254
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by the State Key Program of National Natural Science Foundation of China(No.U1502274), the Innovation Scientists and Technicians Troop Construction Projects of Henan Province (No.C20150014), the Program for Innovation Research Team (in Science and Technology) in University of Henan Province (No.14IRTSTHN007), and the Key Scientific Program of Henan Province(No. 16A430004)
Rights and permissions
About this article
Cite this article
Ji, S., Liang, S., Song, K. et al. Corrosion and electrochemical behavior of Zn-Cu-Ti alloy added with La in 3% NaOH solution. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 31, 408–416 (2016). https://doi.org/10.1007/s11595-016-1384-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11595-016-1384-x