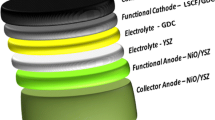
Abstract
Solid oxide fuel cells (SOFCs) are rapidly emerging as a technology, offering the potential for carbon neutral or carbon negative generation of electricity and heat (combined heat and power) using synthetic carbohydrate fuels and hydrogen. A significant challenge associated with SOFCs is the high polarization resistance experienced at the cathode during the oxygen reduction reaction (ORR), which diminishes the cell efficiency. The kinetics of the ORR are influenced by factors such as the cathode material type, its microstructure, and the quality of the interface between the cathode and electrolyte. In our research, we have addressed this issue by modifying the interface between the state-of-the-art cathode material, Lanthanum Strontium Cobalt Ferrite—La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF), and the Scandia stabilized Zirconia (ScSZ) electrolyte. This modification involved the deposition of a micron-sized film of ion-conducting gadolinium-doped ceria (Gd0.1Ce0.9O2-δ) – (GDC) as an interface layer. Our analysis involved systematic studies, including variations in cell operating temperatures and applied potentials, as well as measurements of cell performance over an extended period. We observed a significant enhancement in cell performance with the introduction of the GDC interfacial layer between the LSCF cathode and ScSZ electrolyte. Specifically, we recorded a cathode polarization resistance as low as 0.40 Ωcm2 for the modified interface, which is substantially lower compared to bare LSCF cathodes (3.04 Ωcm2) at 600 °C. This reduction in cathode resistance can primarily be attributed to the improved conditions for the oxygen reduction reaction (ORR), resulting from enhanced interfacial contact between the electrode and the electrolyte and mitigation of any zirconium interdiffusion as seen from detailed scanning electron microscopic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solid oxide fuel cell technology has witnessed remarkable development and scale-up in the past few decades to cope with the gradual shift from fossil fuels to renewable energy. A lot of research and development is still being conducted on different aspects of this technology, and one such area is improving intermediate temperature (500–700 °C) cell kinetics by modifying the electrodes or electrode/electrolyte interfaces. Lanthanum Strontium Manganite—LaxSr1-xMnO3-δ (LSM) is a benchmark cathode material in solid oxide fuel cells (SOFC) for operation at temperatures above 800 °C due to its excellent chemical and thermal stability with Yttria stabilized Zirconia-YSZ (YxZr1-xO3-δ) electrolyte [1]. However, polarization resistance associated with the LSM cathode becomes high at intermediate temperatures due to poor catalytic activity and electronic/ionic conductivity values (electronic conductivity (σe) of 100 S cm−1 and ionic conductivity (σi) of approx. 10−6 S cm−1 at 800 °C) [2]. Therefore, significant efforts are being made to overcome such reduced efficiency of SOFC at a lower temperature range (500–700 °C) due to the sluggish kinetics of the ORR at cathode [3]. In addition to a high electrocatalytic activity for the ORR, a cathode is also required to have thermal and chemical compatibility with other cell components, especially the electrolyte within the operating temperature range. Other factors that influence the cathode performance are the cathode and the cathode/electrolyte interfacial microstructure.
The Strontium (Sr) and Iron (Fe) doped LaCoO3-δ (La1-xSrxCo1-yFeyO3-δ—LSCF) cathode is a promising mixed ionic and electronic conductor (MIEC) with electronic conductivity (σe) of 120 S cm−1 at 500 °C [4], 332 S cm−1 at 600 °C [5], 102 S cm−1 at 800 °C while the ionic conductivity (σi) of 10−2 S cm−1 at 800 °C [6] is favorable for solid oxide fuel cell operation [5, 7]. However, impure phase formation such as non-conductive strontium zirconate (SrZrO3- SZO) and lanthanum zirconate (La2Zr2O7) [with oxygen ion conductivity of 4.2 × 10−4 S cm−1 at 800 °C [8] which is much lower than the state of art electrolyte YSZ (2.5 × 10−2 S cm−1 at 800 °C) [9] and ScSZ (1 × 10−1 S cm−1 at 800 °C) [10] has been widely reported in the literature. As an alternative, there are significant studies on LSCF with doped ceria as a cathode material; however, the thermal mismatch of this material results in material degradation in long-term operation and this issue is still unsolved [11].
The use of ceria-based ceramics as an interlayer between the cathode and the electrolyte is an alternative to control the elemental interdiffusion and cross-reaction between zirconia-based electrolytes and LSCF cathodes. The interlayer materials used are Gd [12] doped or Sm [13] doped ceria between cathode and the electrolyte (i.e., ScSZ and/or YSZ) [14, 15]. Limited research has been conducted to study the effect of doped ceria interlayer deposition at the interface between the cathode and the electrolyte with emphasis on the ORR process at the cathode, despite its active role in oxygen transport and ability to accommodate oxide ions in its structural defects [16, 17]. Structural and thermal stability improvements have been reported by Jung et al. [18] using gadolinium-doped ceria (GDC) as an interfacial layer between ScSZ electrolyte and Zn doped LSCF cathode, which demonstrated the positive impact of interfacial modification on cell performance. Similarly, Kulkarni et al. [19] showed the role of the ultra-thin film of GDC as an interlayer on the enhancement of ORR at the cathode on two different electrolytes (YSZ and SDC) with Pt as the air electrode. GDC is commonly used as a barrier layer between LSCF and YSZ to control SZO (Sr2O3 and/or Zr2O3) formation during sintering and cell operation > 800 °C. In this work, the major focus was on the improvement of cathode performance in the range of 500 °C to 700 °C. Target was to achieve polarization resistance of 0.05 to 0.1 Ωcm2 at 500 °C to 700 °C with interfacial layer modification using low-cost fabrication method.
To investigate the effect of the GDC interlayer on the cathode performance at intermediate temperatures, we have chosen ScSZ as an electrolyte, as it exhibits higher ionic conductivity (1.1 × 10−2 S cm−1 at 600 °C) than YSZ (4.3 × 10−3 S cm−1 at 600 °C) [10].
To the best of our knowledge, this is the first systematic study on the role of the GDC interlayer on the ORR using a LSCF cathode on ScSZ electrolyte-supported cells; this work reports the effect of varying the operating temperature, the duration of testing, and the applied potential on the overall resistance of the cell. This work investigates, for the first time, the improvement in low, middle, and high-frequency processes of cathode because of the incorporation of GDC between LSCF cathode and ScSZ electrolyte in the temperature range of 500 to 700 °C. The electrochemical observations and trends have been further corroborated using detailed scanning electron microscopic studies.
Experimental procedure
Cell fabrication
Symmetrical button cells were fabricated with ScSZ electrolyte disks and LSCF electrodes, and the gadolinium-doped ceria (Gd0.1Ce0.9O2-δ—GDC) interface was formed between the electrolyte and electrode on both sides. For reference, we also fabricated one cell without applying the interfacial layer of GDC. Commercial-grade ScSZ electrolyte disks (Fuel Cell Materials, OH, USA) had a diameter of 20 mm and thickness of 0.14 mm. These disks were washed using an ultrasonic bath and dried in the oven. Next, gadolinium-doped ceria (GDC) ink (Fiaxell) was brush coated on both sides of the electrolyte disks as an interlayer and subjected to heat treatment at 1050 °C for 2 h with heating and cooling rates maintained at 120 °C h−1 to obtain better adhesion of the interlayer with the electrolyte. In this study, the sintering temperature of the GDC interfacial layer (1050 °C) was kept below 1200–1300 °C to eliminate the possibility of the formation of any secondary phases between the ceria and zirconia phases [20], to understand the active role of GDC on the cell performance. The active area was measured to be 0.78 cm2. Commercial-grade LSCF powder (Fuel Cell Materials, OH, USA) [54.5 wt% (3.0 g)] was mixed with [45.5 wt% (2.5 g)] of total weight of ink vehicle (Fuel Cell Materials, OH, USA) and ball-milled for 2 h to make LSCF electrode ink. The as-prepared LSCF ink was brush painted on both sides of the ScSZ electrolyte disks to make bare LSCF / ScSZ / LSCF reference cells (referred to as LSCF-B hereafter) and ScSZ electrolyte disks modified with GDC interlayer to obtain LSCF / GDC / ScSZ / GDC / LSCF (referred to as LSCF-M hereafter) symmetrical button cells. The disks were heat-treated again in a binder burn-off furnace at 775 °C for 2 h at a 120 °C h−1 ramp rate. Finally, silver paste from fuel cell materials was applied as a current collector layer on both sides of the cells and sintered at 775 °C for 2 h with a 120 °C h−1 ramp rate. The symmetrical cells with the above cell configurations were then analyzed using electrochemical impedance spectroscopy (EIS).
Characterization
The commercial-grade LSCF, ScSZ, and GDC powders that were used to prepare LSCF-B and LSCF-M symmetrical cells were fired at 800 °C for 8 h and 100 h in static air. X‐ray diffractograms of the annealed powders were obtained using a Bruker D8 Advance A25 x‐ray Diffractometer operating with CuKα radiation (40 kV, 40 mA) fortified with a Lynx Eye XE‐T detector. The scanning parameters were set as the 2θ range of 5° to 130° with a step size of 0.02° and a count time of 1.6 s per step and were spun at a scanning rate of 15 RPM through the data collection. Phase analyses were performed on the collected XRD data using the Bruker XRD search match program EVA™v6. The ICDD‐JCPDS powder diffraction database was used to identify the crystalline phases.
Next, x-ray diffractograms from as prepared and tested cells were also obtained from the surface as well as from the interface. To get the XRD of the top of as prepared and tested cells a Rigaku SmartLab, with a rotating anode CuKα source (45 kV, 200 mA), and Hypix 3000 detector was used. The diffractometer was operated in Bragg‐Brentano mode with a 1° incidence slit, 20 mm receiving slit, and a beam limiting mask of 5 mm. Data was collected over the 2θ range of 5° to 130° with a step size of 0.01° with a scan rate of 2° per minute. For the XRD at the interface (after scraping the top layers of the samples to study the phases at the interface more intricately), a grazing incidence x‐ray diffractometer was used. The configuration of the diffractometer was done with a beam-limiting mask of 5 mm. Data was collected over the 2θ range of 5° to 115° with a step size of 0.04° and a step rate of 0.5° per minute. Data was obtained with ω set at 0.3°. The beam footprint of ~ 6 mm by ~ 6 mm was maintained by a combination of ω setting, beam limiting mask, and incident slit.
A field emission scanning electron microscope (ZEISS, Merlin series) was used for the microstructural study of the electrode-electrolyte and electrode-interlayer-electrolyte interfaces.
Electrochemical analysis
As-prepared button cells were placed in a tubular furnace at 500 °C with a highly accurate temperature control system in air (ambient testing environment). V-I characteristics and chronoamperometry were performed at a fixed potential of 1 V to study the effect of different physical parameters (temperature and time) and cell operating conditions (OCV and under cathodic polarization). The same set of experiments was repeated at 600 °C and 700 °C for both the LSCF-B and LSCF-M. The electrochemical data were recorded using a Princeton Applied Research VersaSTAT in the frequency range of 1 MHz to 0.1 Hz at 10 mV s−1 amplitude.
Results and discussion
Electrochemical impedance (EIS) analysis
Cell performance under cathodic polarization
The electrochemical performance of LSCF-B and LSCF-M for the ORR were systematically evaluated at three different temperatures for 10 min and 24 h. Figure 1 shows the typical electrochemical impedance spectra (EIS) of LSCF-M and LSCF-B at 500 °C, 600 °C, and 700 °C under OCV. The ohmic resistance (Rohmic) was determined from the first intercept of the high-frequency arc on the x-axis, while the polarization resistance (Rpol) of the electrodes was determined from the difference between the total resistance of the cell (intercept at low frequency) and the ohmic resistance (intercept at high frequency on the real axis). The obtained value was divided by 2 to obtain the cathode polarization since the cells were symmetrical and both anode and cathode were exposed to the same environment [21]. As expected, both the cells showed a decrease in Rpol and Rohmic as an effect of increasing temperature. The ionic conductivity of the electrolyte increases with temperature resulting in a reduced Rohmic [10]. Similarly, an increase in temperature activates the electrodes and reduces resistive losses related to charge-transfer as well as mass-transfer processes of gas adsorption–desorption and diffusion [22], thus resulting in a reduced Rpol as observed here.
As seen from Fig. 1, at all test temperatures, Rohmic is lower for LSCF-B (without GDC interlayer) compared to LSCF-M (with GDC interlayer). It is to be noted here that the 3–5 µm thick GDC layer on either side of the electrolyte would impart an additional Rohmic of 0.11 Ωcm2 at 700 °C [23]. Similar observations have been reported by Hsieh et al. [24] who introduced an electron-blocking layer of ScSZ between a LSCF cathode on GDC electrolyte in a tubular cell while studying the effect of the interface on the performance of microtubular electrolyte-supported SOFC between 650 and 800 °C. At 500 °C and 600 °C, Rpol at OCV increased for LSCF-M. However, at 700 °C, this trend reversed and Rpol dropped from 0.42 Ωcm2 for LSCF-B to 0.18 Ωcm2 for LSCF-M. This could possibly be due to changes in interfacial cation chemistry at the interface at higher temperature. Overall, the resistance decreased with an increase in temperature due to thermal activation, improving the materials’ electrochemical properties such as electronic structure and charge transfer processes. Furthermore, at high-temperature, GDC generates more oxygen vacancies in situ that aids the “oxygen hopping” process across the GDC lattice and at the interface [25]. The OCV testing of both cells after polarization treatment of 10 min and 24 h at 1 V showed improved Rohmic and Rpol when compared to initial OCV values, which indicates that the polarization of the cells activated the cathode and the interfacial layer.
Figure 2 shows the effect of cathodic polarization at 1 V on LSCF-B and LSCF-M at the three testing temperatures (500 °C, 600 °C, and 700 °C). Figure 2a shows the EIS recorded after 10 min of cathodic polarization for LSCF-B and LSCF-M, while 2b shows the EIS recorded after 24 h of chronoamperometry at a constant potential of 1 V for LSCF-B and LSCF-M, respectively. Here we observe an improved performance for LSCF-M compared to LSCF-B at 600 °C and 700 °C under polarization (CV — constant voltage) conditions. For both the samples, EIS at 500 °C did not intercept the x-axis, possibly due to the upper-frequency limits (100 kHz) of the VersaStat and possibly due to enlarged grain boundary/higher resistance of ScSZ at low temperatures of 500 °C. To rule out the possibility of this behavior being related to our samples, we repeated the testing of LSCF-B starting from higher (700 °C) to lower temperature (500 °C) at the same operating conditions [10 min and 24 h under polarization (CV) treatment]. This has been referred to here as testing of LSCF-B in a reverse trend, and the nature of the EIS at 500 °C remained unchanged. Interestingly, we noticed a decrease in Rpol at all temperatures in a reverse trend of testing temperature (700 °C to 500 °C) as compared to the normal trend (500 °C to 700 °C) of LSCF-B (Table 1). Cathode activation at high operating temperatures has already been reported [26].
Electrochemical impedance spectra (EIS) recorded under polarization condition at 1 V for a 10 min, b 24 h with LSCF-B (hollow symbols) and LSCF-M (solid symbols) at 500 °C (red), 600 °C (green), and 700 °C (blue). EIS at 500 °C shows the effect of instrument limitation at a lower testing temperature in both samples under cathodic polarization conditions
Operating the cell at a high temperature (700 °C) may have activated the cathode thus improving cell performance as discussed by Alder et al. [26]. The reason can be alteration in cation compositional changes in the LSCF bulk or at the interface between the LSCF and electrolyte triggered by high operating temperature (700 °C) under polarization.
Next, we studied the effect of cell cathodic polarization for LSCF-B and LSCF-M at 600 °C and 700 ℃. An increased cathode performance has been seen in the past by using GDC as an interlayer between different cathodes and electrolytes [27]. In this study, with the introduction of the GDC interlayer between the LSCF and ScSZ electrolyte, the polarization resistance of the cathode (Rpol) drastically dropped from 3.04 Ωcm−2 to 0.48 Ωcm−2 at 600 °C and from 2.11 to 0.17 Ωcm−2 at 700 °C following 24 h chronoamperometry at 1 V (Table 2).
The enhanced performance can be ascribed to the good adhesion between the layers leading to a better contact, and hence enlarged active area at the cathode/interlayer and interlayer/electrolyte interfaces, as discussed later in the “Scanning electron microscopy and energy dispersive spectroscopy” section [SEM images of LSCF-M in Fig. 9a, b and LSCF – B in Fig. 9c, d]. Wang et al. [28] also reported the structural damage of the interface between LSM cathode and YSZ electrolyte after polarization of the cell. Another explanation for the improved performance of LSCF-M can be the increased ion conduction in the cathode bulk due to the stress developed by the higher oxide ion conductive GDC interfacial layer. GDC electrocatalytic activity is also well known in solid oxide cell operation. Addition of a mixed ionic and electronic conductor promotes the electrochemical activity as has been reported frequently [29]. Coddet et al. [30] discussed the positive effect of barrier layers and stated that the GDC barrier layer can enhance oxygen transport at the cathode side. The stress developed by the underlaying GDC barrier layer is due to structural defects/voids of the GDC and can be beneficial to the transport of oxygen ions compared to the bare cathode.
In the AC Impedance spectroscopy studies, the EIS arcs of the LSCF-B and LSCF-M clearly showed a distinct high frequency (HF) arc and a low frequency (LF) arc (Fig. 2). In LSCF based cathodes, the HF processes are charge transfer processes [31], while the LF processes are mass transfer processes like adsorption/desorption and bulk/surface diffusion. It can be seen in Fig. 2 that there is a significant change in both arcs by introducing the GDC interfacial layer in LSCF-B.
All the processes contribute to the total polarization of the cathode. For LSCF-B, at 600 °C, the polarization resistance increased after 24 h of cell operation. It is evident from Fig. 2 that both the HF and LF arc increased significantly, possibly due to microstructural instability, which increased the charge transfer resistance and associated processes. A similar trend has also been observed for LSCF under polarization for 48 h at 750 °C [27].
Contrarily, in LSCF-M at 600 °C, the contribution of the LF processes to the total Rpol of the cathode is low under both testing conditions of 10 min and 24 h. In this case, as evidenced by HF frequency arc, charge transfer processes are dominating the total polarization resistance of the cathode within 10 min and 24 h as compared to LSCF-B. The performance of the LSCF-M is stable for 24 h under polarization. No significant increase in charge transfer resistance was observed from the HF arc. The reduction in the resistance offered by HF processes in 24 h represents improved charge transfer and related processes and is consistent with increasing stability in current density vs time analysis as shown in Fig. 3.
Rohmic depicted opposite trends for LSCF-B and LSCF-M. For LSCF-B, Rohmic at 700 °C increased post 24 h of operation at constant potential of 1 V, possibly due to Zr interdiffusion and formation of trace amounts of non-conducting phases at the electrode-electrolyte interface as seen from SEM-EDS maps discussed in the “Scanning electron microscopy and energy dispersive spectroscopy” section. In contrast, for LSCF-M, Rohmic at both 600 and 700 °C dropped by more than 10% post 24 h of operation at constant potential of 1 V. The reasons are (1) activation of GDC interlayer resulting in better electronic conductivity, (2) improved thermal stability at the electrode-electrolyte interface resulting in better adhesion and reduced contact losses, and (3) mitigation of any Zr interdiffusion that can otherwise form non-conducting phases at the electrode-electrolyte interface as seen from SEM-EDS maps discussed in the “Scanning electron microscopy and energy dispersive spectroscopy” section.
In conclusion, with the introduction of GDC between the LSCF cathode and the ScSZ electrolyte in LSCF-M, the reaction contributing to the polarization of the cathode is changed from LF processes (mass transfer) to HF (charge transfer) processes, when compared to LSCF-B at both the testing temperatures, i.e., 600 °C and 700 °C.
Short-term stability testing
There is a clear decrease in current density with time for LSCF-B at all testing temperatures (Fig. 3a), especially at high temperatures (700 °C) where it started initially at 256 mA cm−2 and decreased to 219 mA cm−2 in 24 h. The same behavior is observed for 600 °C and 500 °C; however, the decline is not as drastic. At high temperatures, the decrease in current density might be due to a collapse in porosity from catalyst agglomeration and nonconductive phases formation which are common at high temperatures. Wilde et al. [32] documented the non-conductive phase formation at 1100 °C as the sintering temperature, the samples in this study are sintered at 1050 °C with adequate densification [33] which is close to 1100 °C, and the possibility of non-conductive phases cannot be ruled out and has been confirmed from our SEM images as well (discussed in the “Scanning electron microscopy and energy dispersive spectroscopy” section). Another possibility is the voltage-assisted nonconductive phase formation as we have sintered our samples at 1050 °C and no secondary phase formation was detected in XRD. At a lower testing temperature (500 °C), this effect (decrease in stability over 24 h of testing under polarization) is not very prominent, showing the stability of the cathode microstructure under polarization. The reduction in current density at high temperatures could be due to the onset of phase formation of impurities at the interface between the LSCF cathode and the ScSZ electrolyte since high temperature triggers voltage-assisted cross-reactions at interfaces. On the other hand, LSCF-M showed a constant increase in current density with time rather than a decline over 24 h at all testing temperatures (Fig. 3b). This demonstrates the improvement at the interface between the LSCF cathode and ScSZ electrolyte in terms of adhesion, with increased active area or/and enhanced oxygen ion conductivity. A similar effect is shown by Kulkarni et al. [19] who reported the increased active area and better adhesion of the cathode to the electrolyte by introducing the GDC ultra-thin layer at the interface that reduced the Rpol of the cathode. It is obvious from Fig. 3 that the difference between the current density of LSCF-B and LSCF-M is even more significant at higher temperatures (700 °C). This shows the beneficial effect of the GDC interfacial layer on cathode performance at high temperature. The current-voltage characteristics of LSCF-M (Fig. 4a) and LSCF-B (Fig. 4b) at all temperatures also show a clear difference in the behavior of the two electrodes, especially at 600 °C and 700 °C after 24 h chronoamperometry at a constant voltage of 1 V. The LSCF-B shows a mixed reaction mechanism indicated by the two slopes in the V-I curves at the current density of 30 mA cm−2 and 60 mA cm−2 (at 600 °C), and 100 mA cm−2 and 160 mA cm−2 (at 700 °C). This mixed reaction mechanism indicates the contribution of both ohmic and polarization resistances to the performance of the LSCF-B. The behavior (contribution of both ohmic and polarization resistances) becomes dominating at a high testing temperature which is consistent with EIS analysis and stability study. The V-I curve for the LSCF- M shows that the introduction of GDC has reduced the polarization resistance contribution, as indicated by the straight lines at all temperatures, particularly at higher temperatures. Figure 5 shows a comparison of the current-voltage characteristics of LSCF-M and LSCF-B at 600 °C.
Physical characterization
X-rays diffraction
X-ray diffractograms were obtained for the powder precursors of LSCF, ScSz, and GDC mixed in 50–50 wt% (two precursors at a time) and sintered at 800 °C at different time intervals to investigate the formation of secondary phases. Figure 6 shows the XRD spectra of these precursors sintered at 800 °C for 100 h.
X-ray diffractograms obtained from powder precursors sintered at 800 °C for 100 h a LaSrCoFeO3-δ (LSCF) mixed with Scandium stabilized zirconia (ScSZ), b LaSrCoFeO3-δ (LSCF) mixed with Gd-doped Ceria (GDC), and c Scandium stabilized zirconia (ScSZ) mixed with Gd-doped Ceria (GDC). The inset in the figure shows the spectrum of the secondary phase formed in a
No secondary phase formation was noticed in the powder precursors annealed at 800 °C for 8 h in the air. It can be clearly seen that SrZrO3 formation was observed along with some traces of cobalt iron oxide sintering under similar conditions in 100 h (Fig. 6). Sr segregation at higher firing temperature (~ 1200 °C) of the cell is possible and is reported frequently [34]. We report the formation of SrZrO3 even at low firing temperature of powders at 800 °C, over a period of 100 h as compared to 1200 °C. This suggests that non-conducting phase formation can take place at 800 °C when heated for a longer time. Railsback et al. [34] also reported Co interdiffusion from LSCF in GDC layers suggesting the tendency of Co to leach-out from LSCF into other layers. We detected Co oxide in LSCF powder mixed ScSZ powder confirming pass out of Cobalt from LSCF at 800 °C in 100 h (Fig. 6a). We also obtained the XRD diffractograms of the as-prepared and samples tested for 10 min and 24 h. First, we obtained the Bragg‐Brentano diffractograms from the surfaces of the samples, and afterwards, we obtained the Grazing Incident diffractograms after scraping the top layer of the LSCF-B and LSCF-M. The only phases reflected by all diffractograms are pure LSCF, Ag, GDC, and multiple zirconia‐based phases both in as prepared and tested samples. The zirconia-based phases were all detected in the off-the-shelf disks purchased from fuel cell materials while the Ag peak comes from the silver current collector layer on the top. The absence of additional phases in the Grazing incidence obtained from the scraped samples, as well as the Bragg-Brentano diffractograms obtained from top layers of as-prepared and tested cells, confirmed that no inter-phases had formed because of an unwanted reaction between LSCF and ScSZ or LSCF and GDC, suggesting chemical compatibility at cell operating temperatures. The diffractograms collected from chosen regions of as-prepared and tested LSCF-B and LSCF-M samples are given in Fig. 7. The best match phases from the ICDD‐JCPDS powder diffraction database found in these samples are listed in Table 3.
Scanning electron microscopy and energy dispersive spectroscopy
Figure 8 shows the scanning electron microscopy images of the LSCF-M while Fig. 9 shows the electrode–electrolyte and electrode-interlayer-electrolyte interfaces of the LSCF-B and LSCF-M, respectively. These images show cells consisting of a LSCF cathode, ScSZ electrolyte, and the GDC interfacial layer in LSCF-M. It is clear from the SEM images that the LSCF-B has poor contact at the interface between the cathode and electrolyte. In this study, the cathode was sintered on electrolyte at 775 °C to control the high temperature-triggered secondary phase formation. In Fig. 9c and d, the separation of layers/delamination at the interface between LSCF and ScSZ electrolyte can be easily noticed in LSCF-B. This might be due to the lack of enough densification, because of the low firing temperature required for better contact between the layers. This poor contact is causing the loss of active area and structural damage after testing. In contrast, at the same firing temperature (775 °C) of LSCF with GDC layer, the LSCF-M has better contact between the GDC interlayer and LSCF cathode. After testing, there is not much transformation at the cathode/GDC interfacial layer/electrolyte interfaces demonstrating the stability of the interfaces. It is well-known that GDC and ScSZ have similar thermal expansion coefficients (TEC). The TEC values of LSCF, GDC, and ScSZ are 14.6 × 10−6 K−1, 12 × 10−6 K−1 at 700 °C [35] 10.7 × 10−6 K−1 [36], respectively). Since GDC has a TEC value intermediate between LSCF and ScSZ, a GDC interfacial layer improves adhesion between LSCF cathode and ScSZ electrolyte. Such improved adhesion enlarges the active area along the extended triple phase boundary (TPB) which helps to improve the overall performance of the cell. Similar results were reported by Chen et al. [37] and Kulkarni et al. [19]. On the other hand, a poor contact between the LSCF cathode and SSZ electrolyte in tested LSCF-B can be attributed to dissimilar thermal expansion coefficients of LSCF and ScSZ, resulting in non-uniform expansion and delamination at higher operating temperatures and hence poor performance.
The formation of CeZrO2 layer at the GDC/ScSZ interface was investigated through EDS by measuring the percentage quantities of Ce and Zr at various positions at the interface of GDC interfacial layer and ScSZ electrolyte. The EDS results of LSCF-M are depicted in Fig. 10a, b for tested samples and c, d for fresh samples while Fig. 11a, b shows the EDS spectrum of LSCF-B, respectively. Ce/Sr + Zr atomic ratio across the different layers of the samples including LSCF cathode, GDC/ScSZ interface, and ScSZ electrolyte as a function of position was studied. Jung et al. [18] studied the optimization of microstructure of GDC interfacial layer as a function of sintering temperature for the elimination of secondary phase of CeZrO2. However, here we report no CeZrO2 layer formation for GDC/ScSZ interface in both fresh and tested samples of the LSCF-M (Fig. 10a, b and c, d) sintered at 775 ℃ in air. Similarly, no cross-diffusion of any cation from GDC interfacial layer and ScSZ electrolyte in LSCF cathode was detected. A small amount of Sr from LSCF cathode is seen in GDC interfacial layer in LSCF-M in spectrum 2 (Fig. 10a, b). However, in Fig. 11a, b, the EDX analysis of the LSCF-B tested samples shows that diffusion of Zr has taken place in to the LSCF cathode layer [refer to EDS spectrum 2 in Fig. 11a, b). But it can be said that this cross-diffusion of Zr is very small as it was not detected by the detailed analysis through XRD both at the interface and at the surface of the fresh or tested samples. It is proposed that the actual quantity of the Zr diffusion will be much lesser than the width of the cross-diffusion zone in cathode of LSCF-B cell as it was not detected at position 1 and 5 shown in Fig. 11a, b in spectrum 1 and 5, respectively. Clearly, the GDC interlayer is effective in mitigating any cross-diffusion of Zr even in minute quantity as has been envisaged for LSCF-B.
Conclusion
The Electrochemical Impendence Spectroscopic analysis of symmetrical button cell with cell configuration of LSCF | GDC | ScSZ | GDC | LSCF reveals a significant improvement in polarization resistance for the cell with an interlayer between the electrolyte and cathode at all temperatures under cathodic polarization conditions when compared to LSCF | ScSZ | LSCF cells with no interfacial layer modification. Polarization resistance was measured to be 0.48 Ωcm2 at 600 °C and 0.17 Ωcm2 at 700 °C after 24 h of polarization condition at 1 V. The current density improvement at higher temperatures (600 °C and 700 °C) shows the positive impact of the gadolinium-doped ceria as an interfacial layer on the overall performance of cell, possibly due to the improved adherence of cathode to the electrolyte, and possible retardation of cross-reaction between cathode and electrolyte, resulting in an improvement in the oxygen reduction reaction process. The improved performance at 600 °C and 700 °C suggests an induced increase in ionic conductivity of the Lanthanum Strontium Cobalt Ferrite cathode. The potentiodynamic study also revealed a possible change in the oxygen reduction reaction mechanism due to interfacial layer deposition as compared to the bare sample. This suggests that the deposition of the gadolinium-doped ceria interface layer between the Lanthanum Strontium Cobalt Ferrite cathode and Scandium stabilized zirconia electrolyte is not only beneficial for interfacial modification but has an impact on the oxygen reduction reaction mechanism as well.
Data availability
No datasets were generated or analysed during the current study.
References
Morel B, Roberge R, Savoie S, Napporn TW, Meunier M (2007) Appl Catal A 323:181–187
Huber TM, Navickas E, Sasaki K, Yildiz B, Hutter H, Tuller H, Fleig J (2018) J Electrochem Soc 165:F702–F709
Tsai T, Barnett SA (1997) Solid State Ionics 93:207–217
Xia C, Wang B, Ma Y, Cai Y, Afzal M, Liu Y, He Y, Zhang W, Dong W, Li J, Zhu B (2016) J Power Sources 307:270–279
Jiang SP (2019) Int J Hydrogen Energy 44:7448–7493
Teraoka Y, Zhang HM, Okamoto K, Yamazoe N (1988) Mater Res Bull 23:51–58
Burnwal S, Bharadwaj S, Kistaiah P (2016) J Mol Eng Mater 04
Labrincha JA, Frade JR, Marques FMB (1997) Solid State Ionics 99:33–40
Ou G, Liu W, Yao L, Wu H, Pan W (2014) J Mater Chem A Mater Energy Sustain 2:1855–1861
Badwal SPS, Giddey S, Munnings C (2013) Wiley Interdiscip Rev Energy Environ 2:473–487
Anandakumar G, Li N, Verma A, Singh P, Kim J-H (2010) J Power Sources 195:6659–6670
Railsback J, Choi SH, Barnett SA (2019) Solid State Ionics 335:74–81
Milcarek RJ, Wang K, Falkenstein-Smith RL, Ahn J (2016) Int J Hydrogen Energy 41:9500–9506
Nguyen TL, Kobayashi K, Honda T, Iimura Y, Kato K, Neghisi A, Nozaki K, Tappero F, Sasaki K, Shirahama H, Ota K, Dokiya M, Kato T (2004) Solid State Ionics 174:163–174
Park T, Lee YH, Cho GY, Ji S, Park J, Chang I, Cha SW (2015) Thin Solid Films 584:120–124
Srisuwan A, Wattanasiriwech D, Wattanasiriwech S, Aungkavattana P (2013) Suranaree J Sci Technol 20:35–41
Samson Nesaraj A, Arul Raj I, Pattabiraman R (2010) J Iran Chem Soc 7:564–584
Jung DW, Kwak C, Seo S, Moon K-S, Han I-T, Kim JS (2017) J Power Sources 361:153–159
Kulkarni AP, Giddey S, Badwal SPS (2017) J CO2 Util 17:180–187
Liang B, Tao T, Zhang S, Huang Y, Cai Z, Lu S (2016) J Alloy Compd 679:191–195
Samreen A, Galvez-Sanchez M, Steinberger-Wilckens R, Arifin NA, Saher S, Ali S, Qamar A (2020) Int J Hydrogen Energy 45:21714–21721
Liu Y, Zhou F, Chen X, Wang C, Zhong S (2021) J Appl Electrochem 51:1–9
Min Park Y, Kim H (2013) Ceram Int 39:2037–2043
Hsieh W-S, Lin P, Wang S-F (2014) Int J Hydrogen Energy 39:17267–17274
Irvine JTS, Neagu D, Verbraeken MC, Chatzichristodoulou C, Graves C, Mogensen MB (2016) Nat Energy 1:15014
Adler S (2004) Chem Rev 104:4791–4843
Liu Y, Chen K, Zhao L, Chi B, Pu J, Jiang SP, Jian L (2014) Int J Hydrogen Energy 39:15868–15876
Wang S, Jiang Y, Zhang Y, Yan J, Li W (1998) Solid State Ionics 113–115:291–303
Kaur G, Basu S (2013) J Power Sources 241:783–790
Coddet P, Amany M-L, Vulliet J, Caillard A, Thomann A-L (2019) Surf Coat Technol 357:103–113
Wang Z, Sun H, Li J, Guo X, Hu Q, Yang Z, Yu F, Li G (2021) Int J Hydrogen Energy 46:22932–22941
Wilde V, Störmer H, Szász J, Wankmüller F, Ivers-Tiffée E, Gerthsen D (2018) ACS Appl Energy Mater 1:6790–6800
Mengru Z, Lisha A, Enhua W, Hewu W, Minggao O, Haoran H (2022) Catalysts 12:831
Railsback J, Choi SH, Barnett SA (2019) Solid State Ionics 335
Li N, Verma A, Kim J (2013) Ceram Int 39:529–538
Lee SO, Lee D, Jung I, Kim D, Hyun S-H, Kim J, Moon J (2013) J. i. j. o. h. e. , 38:e9329
Chen X, He W, Ding L-X, Wang S, Wang H (2019) Energy Environ Sci 12:938–944
Acknowledgements
We would like to acknowledge Dr Aaron Seeber (CSIRO) for helping with XRD analysis, and Mark Greaves (CSIRO) for helping with SEM.
Funding
Open access funding provided by CSIRO Library Services.
Author information
Authors and Affiliations
Contributions
JN: performed the experimental work, and wrote the manuscript. SG: conceptualised the problem, analysed the data and reviewed the manuscript. GK: helped with experiments, analysed the results. AK: Conceptualised the problem, and analysed the results. SB: helped with experiments, and reviewed the manuscript. LJ: Reviewed the manuscript. SKB: Reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nisar, J., Giddey, S., Kaur, G. et al. The effect of gadolinium-doped ceria interlayer on the oxygen reduction reaction in a LSCF cathode-ScSZ electrolyte supported IT-SOFCs. Ionics (2024). https://doi.org/10.1007/s11581-024-05651-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11581-024-05651-w