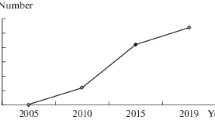
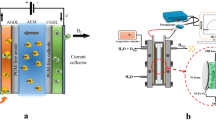
Abstract
Membrane (ion membrane) electrolysis technology has gained a lot of attention and development because of its function and advantage of making full use of the two-stage reaction and separating the products of the two poles. In particular, the membrane electrolysis industry has a great potential to grow in the context of the transformation from “carbon-driven” to “electrically driven.” There are many systems that require membrane or ion membrane electrolysis. Typical ones are electrolytic water to hydrogen, chlor-alkali, electrodialysis, electrometallurgy, etc. In this paper, several typical membrane (ion membrane) electrolysis scenarios are selected and analyzed in detail with respect to their principles, development history, characteristics, problems faced, and development prospects. A theoretical basis is laid for the development and application of efficient industrialized membrane electrolysis technology, which will be beneficial to the technological progress in this field.














Similar content being viewed by others
Data availability
The data in this manuscript has not been published elsewhere and has not been considered by other publishers.
Abbreviations
- IEMs:
-
Ion exchange membranes
- CEMs:
-
Cation exchange membranes
- AWE:
-
Alkaline water electrolysis
- PEM:
-
Proton exchange membrane electrolysis
- AEM:
-
Alkaline ion membrane electrolysis
- SOE:
-
Solid oxide electrolytic
- SOEC:
-
Solid oxide electrolytic cell
- AEMWE:
-
Anion exchange membrane water electrolysis
- YSZ:
-
Y2O3-stabilized ZrO2
- ScSZ:
-
Scandium-stabilized zirconium oxide
- LSM:
-
La1-xSrxMnO3
- HP:
-
Hydrogen production
- PFSA:
-
Perfluorosulfonic acid membrane
- PFCA:
-
Perfluoro carboxylic acid membrane
- PTFE:
-
Polytetrafluoroethylene
- ED:
-
Electrodialysis
- SCT-SAPs:
-
Side chain sulfonated aromatic polymers
- R s :
-
Measured internal resistance of the membrane [Ω]
- A :
-
Area of the electrode [cm2]
- R :
-
R = 8.314 J/(mol·K) is the universal gas constant
- T :
-
Reaction temperature [K]
- F :
-
Faraday’s constant [C·mol−1]
- φ m :
-
Membrane potential
- φ s :
-
Solution potential
- σ :
-
Porosity
References
Madaleno M, Dogan E, Taskin D (2022) A step forward on sustainability: the nexus of environmental responsibility, green technology, clean energy and green finance. Energy Econ 109:105945
Allan G, McGregor P, Swales K (2017) Greening regional development: employment in low-carbon and renewable energy activities. Reg Stud 51(8):1270–1280
Wu LT (1999) Characteristics of ionic membrane and its application in chlor-alkali production. J Pet Technol 9(2):84–90
Crutzen PJ, Brauch HG (2016) A pioneer on atmospheric chemistry and climate change in the Anthropocene, vol 50. Springer
Zalasiewicz J, Williams M (2011) The Anthropocene: a new epoch of geological time? Philosophical Transactions of the Royal Society A: Mathematical. Phys Eng Sci 369(1938):835–841
Zhang X, Hu J, Cheng X (2021) Double metal-organic frameworks derived Fe-Co-Ni phosphides nanosheets as high-performance electrocatalyst for alkaline electrochemical water splitting. Electrochim Acta 367:137536
Jin H, Guo C, Liu X (2018) Emerging two-dimensional nanomaterials for electrocatalysis. Chem Rev 118(13):6337–6408
Kander A, Malanima P, Warde P (2014) Power to the people. In: Power to the people. Princeton University Press
Adom PK, Amuakwa-Mensah F, Agradi MP (2021) Energy poverty, development outcomes, and transition to green energy. Renew Energy 178:1337–1352
Stern DI, Burke PJ, Bruns SB (2019) The impact of electricity on economic development. In: A Macroeconomic Perspective
Alam MS, Miah MD, Hammoudeh S (2018) The nexus between access to electricity and labour productivity in developing countries. Energy Policy 122:715–726
Leng Y, Chen G, Mendoza AJ (2012) Solid-state water electrolysis with an alkaline membrane. J Am Chem Soc 134(22):9054–9057
Tan Y, Yang H, Cheng J (2022) Preparation of hydrogen from metals and water without CO2 emissions. Int J Hydrog Energy 47(90)
Shi J (2001) Membrane technical handbook. Chemical Industry Press
Hoek E, Tarabara VV. Encyclopedia of membranescience and technology (2013).
Meyer KH, Straus W (1940) La permeabilite des membranes VI. Sur le passage du courant electrique a travers des membranes sélectives. Helv Chim Acta 23(1):795–800
Nishiwaki T (1972) Concentration of electrolytes prior to evaporation with an electromembrane process. In: Industrial Processing with Membranes, pp 83–106
Grot W. (1973) Laminates of support material and fluorinated polymer containing pendant side chains containing sulfonyl groups. U.S. Patent No. 3,770,567
Paidar M, Fateev V, Bouzek K (2016) Membrane electrolysis—history, current status and perspective. Electrochim Acta 209:737–756
Pourcelly G, Gavach C (2000) Electrodialysis water splitting-application of electrodialysis with bipolar membranes. Handbook on bipolar membrane technology, vol 17. Twente University Press, Enschede
Kogure M, Ohya H, Paterson R (1997) Properties of new inorganic membranes prepared by metal alkoxide methods Part II: new inorganic-organic anion-exchange membranes prepared by the modified metal alkoxide methods with silane coupling agents. J Membr Sci 126(1):161–169
Xu T (2005) Ion exchange membranes: state of their development and perspective. J Membr Sci 263(1-2):1–29
Daufin G, Escudier JP, Carrere H (2001) Recent and emerging applications of membrane processes in the food and dairy industry. Food Bioprod Process 79(2):89–102
Tarvainen T, Svarfvar B, Akerman S (1999) Drug release from a porous ion-exchange membrane in vitro. Biomaterials 20(22):2177–2183
Kim YH, Moon SH (2001) Lactic acid recovery from fermentation broth using one-stage electrodialysis. J Chem Technol Biotechnol: Int Res Process Environ Clean Technol 76(2):169–178
Guido S (2003) Ionic membrane technologies for the recovery of valuable chemicals from waste waters. Ann Chim 93(9-10):817–826
Bazinet L, Lamarche F, Ippersiel D (1998) Bipolar-membrane electrodialysis: applications of electrodialysis in the food industry. Trends Food Sci Technol 9(3):107–113
Zuo P, Ziang X, Zhu Q (2022) Ion exchange membranes: constructing and tuning ion transport channels. Adv Funct Mater 32(52)
Wang FY (2001) History of organic electrochemistry. Chemistry Education
Lagadec MF, Zahn R, Wood V (2019) Characterization and performance evaluation of lithium-ion battery separators. Nat Energy 4(1):16–25
Wang SF (2000) Theoretical modification of ion migration pathways and selective permeability. J Lanzhou Railway Inst 19(3):70–74
Donnan FG (1924) The theory of membrane equilibria. Chem Rev 1(1):73–90
Donnan FG (1934) Die genaue Thermodynamik der Membrangleichgewichte. II. Zeitschrift fur Physikalische Chemie 168(1):369–380
Hanai T (1981) Membrane and ions theory and calculation of mass transport, pp 221–244
Tanaka Y (2015) Ion exchange membranes: fundamentals and applications. Elsevier
Wu S, Xiao R, Li H (2022) New insights into the mechanism of cation migration induced by cation–anion dynamic coupling in superionic conductors. J Mater Chem A 10(6):3093–3101
Zhao H, Yuan ZY (2023) Progress and perspectives for solar-driven water electrolysis to produce green hydrogen. Adv Energy Mater:2300254
Huang Y, Gong Q, Song X (2016) Mo2C nanoparticles dispersed on hierarchical carbon microflowers for efficient electrocatalytic hydrogen evolution. ACS Nano 10(12):11337–11343
Yin J, Fan Q, Li Y (2016) Ni-C-N nanosheets as catalyst for hydrogen evolution reaction. J Am Chem Soc 138(44):14546–14549
Yu C, Han X, Liu Z (2018) An effective graphene confined strategy to construct active edge sites-enriched nanosheets with enhanced oxygen evolution. Carbon 126:437–442
Adabi H, Shakouri A (2021) High-performing commercial Fe-N-C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat Energy 6(8):834–843
Zhan T, Bie R, Shen Q, Lin L, Wu A, Dong P (2020) Application of electrolysis water hydrogen production in the field of renewable energy power generation. IOP Conference Series: Earth and Environmental Science 598(1):012088
Vincent I, Bessarabov D (2018) Low cost hydrogen production by anion exchange membrane electrolysis: a review. Renew Sustain Energy Rev 81:1690–1704
Wan L, Xu ZA, Wang PC (2021) Progress of alkaline-resistant ion membranes for hydrogen production by water electrolysis. Chemical Industry and Engineering Progress 6161–6175
Li C, Baek JB (2021) The promise of hydrogen production from alkaline anion exchange membrane electrolyzers. Nano Energy 87:106162
Thomas D. (2019) Large scale PEM electrolysis: technology status and upscaling strategies. 8 October 2019. http://hybalance.eu/wp-content/uploads/2019/10/Large-scale-PEM-electrolysis.pdf.
Abbasi R, Setzler BP, Lin S (2019) A roadmap to low-cost hydrogen with hydroxide exchange membrane electrolyzers. Adv Mater 31(31):1805876
Tunold R, Marshall AT, Rasten E, Tsypkin M, Owe L-E, Sunde S (2010) Materials for electrocatalysis of oxygen evolution process in pem water electrolysis cells. ECS Trans 25(23):103–117. https://doi.org/10.1149/1.3328515
Chauvy R, Dubois L, Lybaert P (2020) Production of synthetic natural gas from industrial carbon dioxide. Appl Energy 260:114249
Xue FM, Su JC, Li PP (2021) Application of proton exchange membrane electrolysis of water hydrogen production technology in power plant. IOP Conference Series Earth and Environmental Science 631:012079
Wang PC (2021) Hydrogen production based-on anion exchange membrane water electrolysis: a critical review and perspective. J Chem Ind Eng
Zhang J, Ren LB, Li YH, Xu ZB (2008) Technical progress of proton exchange membrane water electrolyzer. Power Technol 32(4):261–265
Goni-Urtiaga A, Presvytes D, Scott K (2012) Solid acids as electrolyte materials for proton exchange membrane (PEM) electrolysis. Int J Hydrog Energy 37(4):3358–3372
Mi WL, Jun FR (2021) Progress and application prospects of PEM water electrolysis technology for hydrogen production. Petroleum processing and petrochemicals 52(10):78
Carmo M, Fritz DL, Mergel J (2013) A comprehensive review on PEM water electrolysis. Int J Hydrogen Energy 38:4901–4934
Kumar SS, Himabindu V (2019) Hydrogen production by PEM water electrolysis–a review. Mater Sci Energy Technol 2(3):442–454
Ganci F, Lombardo S, Sunseri C (2018) Nanostructured electrodes for hydrogen production in alkaline electrolyzer. Renew Energy 123:117–124
Zhang W, Yu B, Chen J (2008) Hydrogen production through solid oxide electrolysis at elevated temperatures. Prog Chem
Jacobs JH, Hunter JW, Yaroll WH (1946) Operations of electrolytic manganese pilot plant at Boulder City. Technical Report Archive & Image Library
Chen T, Wang SR (2014) Water electrolysis using SOECs: A review. Journal of Ceramics
Maskalick NJ (1986) High temperature electrolysis cell performance characterization. Int J Hydrogen Energy 11(9):563–570
Spacil HS, Tedmon CS (1969) Electrochemical dissociation of water vapor in solid oxide electrolyte cells: II. Materials, fabrication, and properties. J Electrochem Soc 116(12):1627
Donitz W, Erdle E (1985) High-temperature electrolysis of water vapor—status of development and perspectives for application. Int J Hydrogen Energy 10(5):291–295
Brien JE, Stoots CM, Herring JS (2005) Performance measurements of solid-oxide electrolysis cells for hydrogen production, pp 156–163
Ni M, Leung MKH, Leung DYC (2008) Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int J Hydrogen Energy 33(9):2337–2354
Tsipis EV, Kharton VV (2011) Electrode materials and reaction mechanisms in solid oxide fuel cells: a brief review. III. Recent trends and selected methodological aspects. J Solid State Electrochem 15(5):1007–1040
Laguna-Bercero MA (2012) Recent advances in high temperature electrolysis using solid oxide fuel cells: a review. J Power Sources 203:4–16
Kamlungsua K, Su PC, Chan SH (2020) Hydrogen generation using solid oxide electrolysis cells. Fuel Cells 20(6):644–649
Du YC, Lei H, Qian YH (2021) Hydrogen production technology and development status of electrolyzed water. In: Shanghai Energy Saving
Mao Z (2015) Thoughts on chlor alkali production process. Chemical Management 14:183–183
Ito H, Manabe A (2022) Chlor–alkali electrolysis. Electrochemical Power Sources: Fundamentals, Systems, and Applications. pp 281–304
Japan Soda Industry Association. Centurial soda industry in Japan. (1982).
Qiao XF, Guo J, Liu X (2021) Structure and process control principle of ion membrane electrolyzer. Chlor-alkali Industry 12(57):10–20
Yue WT, Liu XM, Liu GZ (2015) Effect of current density on the transfer characteristics of chlor-alkali industrial ion membrane electrolyzers. Ciesc J 66(3):915–923
Li YK (2011) Problems and countermeasures in membrane caustic soda production in chlor alkali enterprises. Safety Health and Environ 11(5):52–53
Chen DS (1998) Study on treatment of wastewater by membraneseparation technology. Membr Sci Technol 18(5):32–34
Scarazzato T, Panossian Z (2017) A review of cleaner production in electroplating industries using electrodialysis. J Clean Prod 168:1590–1602
Juda W, McRae WA (1950) Coherent ion-exchange gels and membranes. J Am Chem Soc 72(2):1044–1044
Mohammadi T, Razmi A, Sadrzadeh M (2004) Effect of operating parameters on Pb2+ separation from wastewater using electrodialysis. Desalination 167:379–385
Hua HL, Wu GX, Liu K (2001) New advances in electrodialysis technology. Environ Pollution Control Technol Equip 2(3):44
Xing GL, Wang XY, Zhao H (2006) Research progress of electrodialysis membrane fouling. Salt and Chem Industry 35(6):42–46
Min KJ, Kim JH, Park KY (2021) Characteristics of heavy metal separation and determination of limiting current density in a pilot-scale electrodialysis process for plating wastewater treatment. Sci Total Environ 757:143762
Zhao Y, Li Y, Yuan S (2019) A chemically assembled anion exchange membranesurface for monovalent anion selectivity and fouling reduction. J Mater Chem A 7(11):6348–6356
Bazinet L, Geoffroy TR (2020) Electrodialytic processes: market overview, membrane phenomena, recent developments and sustainable strategies. Membranes 10(9):221
Zhang WR, Fan X (2009) Electrodialysis concentrates seawater to produce salt. Water Treat Technol 35(2):1–4
Wang HG, Wang XJ (2017) Research progress of electrodialysis seawater desalination technology. Guangdong Chem Indus 44(20):138–140
Osborn CS. Electrodeposition of iron: US884075. 1908.
Boucher A. (1914) Process for the industrial manufacture of electrolytic iron: US1086132
Gao CJ, Ruan GL (2016) Seawater desalination technology and engineering. Chemical Industry Press, Beijing
Peng FB, Jiao XN (2008) Development and application of new diaphragms used in electrolyser for hydrogen preparation. Journal of Textile Research
Gonzalez A, Grageda M, Ushak S (2017) Assessment of pilot-scale water purification module with electrodialysis technology and solar energy. Appl Energy 206:1643–1652
Strathmann H (2010) Electrodialysis, a mature technology with a multitude of new applications. Desalination 264(3):268–288
Yang J, Tang C, Yang S (2009) The separation and electrowinning of bismuth from a bismuth glance concentrate using a membrane cell. Hydrometallurgy 100(1-2):5–9
Zeng W (2006) Problems and countermeasures in the export of electrolytic manganese. China Manganese Indus 24(3):19–20
Liu B, Lyu K, Chen Y (2020) Energy efficient electrodeposition of metallic manganese in an anion-exchange membrane electrolysis reactor using Ti/IrO2–RuO2–SiO2 anode. J Clean Prod 258:120740
Lu J, Dreisinger D, Gluck T (2014) Manganese electrodeposition—a literature review. Hydrometallurgy 141:105–116
Yu XZ, Li NX (2017) Research on purification ofmanganese sulfate in electrolytic manganese industry. Inorganic Chemicals Industry
Huang J, Du HW, Chen G (2020) Analysis of electrolytic manganese current efficiency control mechanism of integrated membrane assembly. Appl Chem 49(2):61–62
Duan N, Fan W, Changbo Z (2010) Analysis of pollution materials generated from electrolytic manganese industries in China. Resour Conserv Recycl 54(8):506–511
Jiao P, Xu F, Li J (2016) The inhibition effect of SeO2 on hydrogen evolution reaction in MnSO4-(NH4)2SO4 solution. Int J Hydrogen Energy 41(2):784–791
Fan X, Xi S, Sun D (2012) Mn-Se interactions at the cathode interface during the electrolytic-manganese process. Hydrometallurgy 127:24–29
Mostad E, Rolseth S, Thonstad J (2008) Electrowinning of iron from sulphate solutions. Hydrometallurgy 90(2-4):213–220
Badenhorst WD, Rossouw C, Cho H (2019) Electrowinning of iron from spent leaching solutions using novel anion exchange membranes. Membranes 9(11):137
Zhu QS (2022) Analysis of ultra-low-carbon ironmaking technology path. Prog Chem 41(3):1391–1398
Cadarelli F (2011) Electrochemical method for recovering useful materials of metallic iron and sulfuric acid from iron-rich sulfate waste, mining residues and pickling liquids. AT503864T, 2011. CN102084034A
Pham AQ, Nijhawan S, Alvarez A (2022) Iron conversion system and applications. WO2022204387A1.
Funding
The National Natural Science Foundation of China (No. 51504231), State Key Laboratory of Multiphase Complex Systems (No. MPCS-2022-A-02), Yunnan Ten Thousand Talents Plan Young & Elite Talents Project (YNWR-QNBJ-2018-327), and Innovation Academy for Green Manufacture Institute, Chinese Academy of Sciences (No. IAGM2022D08).
Author information
Authors and Affiliations
Contributions
HS: wrote the original draft, revised the manuscript, and conducted investigation. HY (corresponding author): conceptualization, project administration, and framework of the manuscript. XY: provided some ideas and discussions. XW: provided some ideas and discussions. HJ: provided some ideas and discussions. YT: provided some ideas and discussions. JH: provided some ideas and discussions. All authors have reviewed the manuscript and agreed to publish it.
Corresponding authors
Ethics declarations
Ethical approval
This article does not involve any biological experiments.
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, H., Yang, H., Yu, X. et al. Research progress of industrial application of membrane electrolysis technology. Ionics 30, 1223–1243 (2024). https://doi.org/10.1007/s11581-024-05395-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-024-05395-7