Abstract
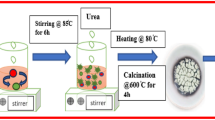
In this study, multifunctional CoFe2O4 and SiO2-CoFe2O4 nanocomposites were synthesized using a thermal decomposition method and used as an electrode material for supercapacitors. SiO2 nano matrix was synthesized from coal fly ash (Tuticorin Thermal Power Plant) and embedded with spinel metal ferrites to produce SiO2-CoFe2O4 nanocomposites with outstanding electrochemical performance. The synthesized composite materials were characterized by analytical methods. From the X-ray diffractometer, amorphous characteristics were observed for the synthesized nanocomposites. The obtained SiO2-CoFe2O4 nanocomposite showed a surface area of 756.4 m2g−1, with a large pore volume which is favorable for charge storage properties. The vibrations of metal ions and the silanol-siloxane bonds are detected using Fourier-transform infrared spectrographs. Spectroscopic and microscopic studies confirmed the formation of SiO2-CoFe2O4 nanocomposites in which the SiO2 nano matrix is encapsulated with CoFe2O4 nanocomposites. According to diffuse reflectance spectroscopy, the bandgap of SiO2-CoFe2O4 (3.3 eV) nanocomposites was found to be smaller than that of pristine SiO2 (3.8 eV) and CoFe2O4 (3.4 eV) nanoparticles, which led to a higher conductivity suitable for increased capacitance. Galvanostatic charge–discharge, cyclic voltammetry, and electrochemical impedance spectroscopy were used to analyze their electrochemical behavior. The specific capacitance of the composite materials was higher than that of the pristine, with SiO2-CoFe2O4 spinel metal oxide nanocomposites having a specific capacitance of 1259.4 Fg−1 at 1 A/g. In addition, the nanocomposites exhibit pseudo-capacitance with excellent cyclic stability of 89.9% capacitance retention for 5000 cycles. The power law provides a qualitative analysis of the diffusivity of the nanocomposites at the electrode/electrolyte interface. The fabricated SiO2-CoFe2O4 electrode showed a stronger reduction in the Rct value than the pristine nanocomposites. The materials have demonstrated promising potential as electrodes for energy conversion and storage.


















Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Rani B, Sahu NK (2020) Electrochemical properties of CoFe2O4 nanoparticles and its rGO composite for supercapacitor. Diam Relat Mater 108:107978. https://doi.org/10.1016/j.diamond.2020.107978
Hongyan G, Shuai L, Yafei L, Eric C, Yan C (2017) A critical review of spinel structured iron cobalt oxides based materials for electrochemical energy storage and conversion. Energies 10(11):1787. https://doi.org/10.3390/en10111787
Xia C, Ren T, Darabi R, Nooshabadi MS, Karaman C, Karimi F (2023) Spotlighting the boosted energy storage capacity of CoFe2O4/graphene nanoribbons: a promising positive electrode material for high-energy-density asymmetric supercapacitor. Energy 270:126914. https://doi.org/10.1016/j.energy.2023.126914
Chhavi K M, Khaiwal R, Suman M (2017) Application of sol–gel technique for preparation of nanosilica from coal-powered thermal power plant fly ash. J Sol-Gel Sci Technol 83. https://link.springer.com/article/10.1007/s10971-017-4440-x
Sekhar S C, Lee J H, Eun-Bum C, Jae S Y (2021) Unveiling redox-boosted mesoporous Co@NiO-SiO2 hybrid composite with hetero-morphologies as an electrode candidate for durable hybrid supercapacitors. J Mater Res Technol 13(35). https://doi.org/10.1016/j.jmrt.2021.05.104
Malarvizhi M, Meyvel S, Sandhiya M, Sathish M, Dakshana M, Sathya P (2021) Design and fabrication of cobalt and nickel ferrites based flexible electrodes for high-performance energy storage applications. Inorg Chem Commun 123:108344. https://doi.org/10.1016/j.inoche.2020.108344
Lee C, Chang H, Jang H D (2018) Preparation of CoFe2O4-graphene composites using aerosol spray pyrolysis for supercapacitors application. Aerosol Air Qual Res 19(3). https://doi.org/10.4209/aaqr.2018.10.0372
Zhang C, Bhoyate S, Zhao C, Pawan KK, Kostoglou N, Mitterer C (2019) Electrodeposited nanostructured CoFe2O4 for overall water splitting and supercapacitor applications. Catalysts 9(2):176. https://doi.org/10.3390/catal9020176
Ahmed H (2021) Synthesis of SiO2/CoFe2O4 Nanoparticles doped CMC: exploring the morphology and optical characteristics for photodegradation of organic dyes. J Inorg Organometal Polym Mater 31(31):1–9. https://link.springer.com/article/10.1007/s10904-020-01846-6
Avazpour L, Toroghinejad MR, Shokrollahi H (2016) Enhanced magneto-optical Kerr effect in rare earth substituted nanostructured cobalt ferrite thin film prepared by sol–gel method. Appl Surf Sci 387(2016):869–874. https://doi.org/10.1016/j.apsusc.2016.06.168
Kiran VS, Sumathi S (2017) Comparison of catalytic activity of bismuth substituted cobalt ferrite nanoparticles synthesized by combustion and co-precipitation method. J Magn Magn Mater 421:113–119. https://doi.org/10.1016/j.jmmm.2016.07.068
Sodaee T, Ghasemi A, Razavi RS (2016) Controlled growth of large-area arrays of gadolinium substituted cobalt ferrite nanorods by hydrothermal processing without use of any template. Ceram Int 42:17420–17428. https://doi.org/10.1016/j.ceramint.2016.08.042
Amiri M, Niasari MS, Pardakhty A, Ahmadi M (2017) Caffeine, A novel green precursor for the synthesis of magnetic CoFe2O4 nanoparticles and pH-sensitive magnetic alginate beads for drug delivery. Mater Sci Eng C 76:1085–1093. https://doi.org/10.1016/j.msec.2017.03.208
El-Sheikh SM, Harraz FA, Hessien MM (2010) Magnetic behavior of cobalt ferrite nanowires prepared by template-assisted technique. Mater Chem Phys 123:254–259. https://doi.org/10.1016/j.matchemphys.2010.04.005
Kishore PNR, Jeevanandam P (2012) A novel thermal decomposition approach for the synthesis of silica-iron oxide core-shell nanoparticles. J Alloy Compd 522:51–62. https://doi.org/10.1016/j.jallcom.2012.01.076
Ayman I, Rasheed A, Sara A, Abdul R, Awais A, Imran S et al (2020) CoFe2O4 Nanoparticles-decorated 2D MXene: a novel hybrid material for supercapacitors applications. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.0c00959
Yujin L, Cuimeng S, Jinchao C, Xueni S, Jinping C, Yun L et al (2020) Sulfur and nitrogen Co-doped activated CoFe2O4@C nanotubes as an efficient material for supercapacitor applications. Carbon 162:124–135. https://doi.org/10.1016/j.carbon.2020.02.050
Debika G, Manash RD, Narendra NG (2022) CoFe2O4 hollow spheres- decorated three-dimensional rGO sponge for highly efficient electrochemical charge storage devices. ACS Omega 7(13):11305–11319. https://doi.org/10.1021/acsomega.2c00374
Qing W, Haoran G, Xuefeng Q, Jianfeng D, Weixue L (2020) Fabrication of NiFe2O4@CoFe2O4 core-shell nanofibers for high-performance supercapacitors. Mater Res Express 7(1):015020. https://doi.org/10.1088/2053-1591/ab61ba
Ping L, Hongyan XC, Yuan X, Jiawei Z, Zhicheng Z, Hualin L et al (2017) Novel method of preparing CoFe2O4/graphene by using steel rolling sludge for supercapacitor. Electrochim Acta 231:565–574. https://doi.org/10.1016/j.electacta.2017.02.088
Hutlove A, Niznansky D, Plocek J et al (2003) J Sol-Gel Sci Techn 26(2003):473-477
Nayak PP, Datt AK (2020) Synthesis of SiO2-nanoparticles from rice husk ash and its comparison with commercial amorphous silica through material characterization. Silicon 13(1). https://doi.org/10.1007/s12633-020-00509-y
Tamilselvi R, Lekshmi GS, Vaithilingam S, Olha B, Igor L, Kateryna B et al (2022) Spinel CoFe2O4 Nanoflakes: a path to enhance energy generation and environmental remediation potential of waste-derived rGO. J Nanomater 12(21):3822. https://doi.org/10.3390/nano12213822
Shipa MA, Nagaswarupa HP, Anil MR et al (2020) Sonochemical synthesis of NiFe2O4 nanoparticles: characterization and their photocatalytic and electrochemical applications. J Appl Surf Sci Adv 1:100023. https://doi.org/10.1016/j.apsadv.2020.100023
Mourad E, Coustan L, Lannelongue P, Zigah D et al (2017) Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat Mater 16(4):446–453. https://doi.org/10.1038/nmat4808
Kiran Babu L, Reddy RYV (2020) Synthesis and characterization of magnetically core-shell structured CoFe2O4/SiO2 nanoparticles; their enhanced antibacterial and electrocatalytic properties. Colloids Surf A: Physicochem Eng Asp 598:124806. https://doi.org/10.1016/j.colsurfa.2020.124806
Carla C, Anna M, Andrea A, Federica O, Davide P, Mariano C et al (2010) CoFe2O4 and CoFe2O4/SiO2 core/shell nanoparticles: magnetic and spectroscopic study. Chem Mater 22(11):3353–3361. https://doi.org/10.1021/cm903837g
Shan F, Wen W, Hua K, Yu Z (2018) Facile synthesis of morphology controllable CoFe2O4 particles as high-performance electrode materials. Part Part Syst Charat 35(10). https://doi.org/10.1002/ppsc.201800223
Mohammad S R, Maryam H, Abolhassan N, Maher F. El-K, Mir F. M, Richard B. K (2019) Asymmetric supercapacitors: an alternative to activated carbon negative electrodes based on earth-abundant elements. Mater. Today Energy 12. https://www.sciencedirect.com/science/article/pii/S2468606918302843?dgcid=rss_sd_all
Priyanka M, Narendra NG (2020) Facile synthesis of MnFe2O4 hollow sphere-reduced graphene oxide nanocomposite as electrode material for all-solid-state flexible high-performance asymmetric supercapacitor. ACS Appl Energy Mater 3(3):2653–2664. https://doi.org/10.1021/acsaem.9b02360
Gourav B, Sam JF (2019) Utilization of mechanically activated waste red mud as a high-performance pseudocapacitor electrode material. Glob Chall 3(2):1800066. https://doi.org/10.1002/gch2.201800066
Sapna NB, Vinod K, Singh SK (2018) Synergistic effect in structural and supercapacitor performance of well dispersed CoFe2O4/Co3O4 nano-heterostructures. Ceram Int 44(12):13806–13814. https://doi.org/10.1016/j.ceramint.2018.04.224
Naresh R M and Sankara S V T (2020) Electrochemical behavior of cobalt oxide/boron incorporated reduced graphene oxide nanocomposite electrode for supercapacitor applications. J Mater Eng Perform 29(10):6535–6549. https://ui.adsabs.harvard.edu/link_gateway/2020JMEP...29.6535N/doi:10.1007/s11665-020-05176-z
Lingxia Z, Lingtong G, Guang Y, Sanming C, Huajun Z (2018) One-pot synthesis of CoFe2O4/rGO hybrid hydrogels with 3D networks for high capacity electrochemical energy storage devices. RSC Adv 8(16):8607–8614. https://doi.org/10.1039/C8RA00285A
Toghan A, Khairy M, Kamar EM, Mousa MA (2022) Effect of particle size and morphological structure on the physical properties of NiFe2O4 for supercapacitor application. J Market Res 19:3521–3535. https://doi.org/10.1016/j.jmrt.2022.06.095
Acknowledgements
We are very grateful to the Department of Physics and Chemistry V.O. Chidambaram College, Tuticorin, for providing us with the lab facilities to carry out our research work.
Author information
Authors and Affiliations
Contributions
Infant Francita Fonseka Christopher: conceptualization, methodology, writing – original draft. Amudhavalli Karuppiah: validation, investigation, review and editing, correction proof. Vinoline Golda Thanapalan: review and editing.
Corresponding author
Ethics declarations
Consent to participate
All authors have given consent to participate.
Consent for publication
All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Christopher, I.F.F., Karuppiah, A. & Thanapalan, V.G. Fabrication of silica-cobalt ferrite (SiO2-CoFe2O4) nanocomposites for high electrochemical performances. Ionics 29, 5055–5072 (2023). https://doi.org/10.1007/s11581-023-05230-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05230-5