Abstract
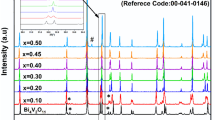
While BIMEVOX systems have attracted the attention of researchers for their electrical conductivity by O2− oxide ions at relatively low temperatures, there is only a limited number of works concerning their local structure. In this work, the Bi4V1.7(Si.Me)0.3O11-δ (Me = Si, P, Cu, and Co) system is studied using X-ray powder diffraction (XRD), Raman spectroscopy, IR spectroscopy, SEM–EDX, UV–visible spectrophotometry, and differential scanning calorimetry (DSC). The three main polymorphs α, β, and γ are obtained at room temperature. In the case of the Bi4Si0.15P0.15V1.70O11-δ compound, two successive structural transitions were observed, while only one structural transition was observed for the Bi4Si0.30V1.70O11-δ compound. The UV–vis diffuse reflectance spectroscopy (DRS) indicates that the double-doped Bi4V1.7(Si.Me)0.3O11-δ compounds present a band gap energy in the range 1.76 ≤ Eg ≤ 2.36 eV and Bi4Si0.15Co0.15V1.70O11-δ presents the narrowest band gap.







Similar content being viewed by others
Data Availability
Data and materials availability.
References
Abraham F, Debreuille-Gresse MF, Mairesse G, Nowogrocki G (1988) Phase transitions and ionic conductivity in Bi4V2O11 an oxide with a layered structure. Solid State Ionics 28–30:529–532. https://doi.org/10.1016/S0167-2738(88)80096-1
Boivin J (1998) Electrode-electrolyte BIMEVOX system for moderate temperature oxygen separation. Solid State Ionics 113–115:639–651. https://doi.org/10.1016/S0167-2738(98)00330-0
Pirovano C, Vannier RN, Nowogrocki G et al (2003) Characterisation of the electrode-electrolyte BIMEVOX system for oxygen separation: part II. Thermal studies under controlled atmosphere. Solid State Ionics 159:181–191. https://doi.org/10.1016/S0167-2738(03)00078-X
Lacorre P, Goutenoire F, Bohnke O et al (2000) Designing fast oxide-ion conductors based on La2Mo2O9. Nature 404:856–858. https://doi.org/10.1038/35009069
Iharada T, Hammouche A, Fouletier J et al (1991) Electrochemical characterization of BIMEVOX oxide-ion conductors. Solid State Ionics 48:257–265. https://doi.org/10.1016/0167-2738(91)90040-I
Abraham F, Boivin J, Mairesse G, Nowogrocki G (1990) The bimevox series: a new family of high performances oxide ion conductors. Solid State Ionics 40–41:934–937. https://doi.org/10.1016/0167-2738(90)90157-M
Abrahams I (2003) A model for the mechanism of low temperature ionic conduction in divalent-substituted γ-BIMEVOXes. Solid State Ionics 157:139–145. https://doi.org/10.1016/S0167-2738(02)00201-1
Mairesse G, Roussel P, Vannier RN et al (2003) Crystal structure determination of α, β and γ-Bi4V2O11 polymorphs. Part I: γ and β-Bi4V2O11. Solid State Sci 5:851–859. https://doi.org/10.1016/S1293-2558(03)00015-3
Mairesse G, Roussel P, Vannier RN et al (2003) Crystal structure determination of α-, β- and γ-Bi4V2O11 polymorphs. Part II: crystal structure of α-Bi4V2O11. Solid State Sci 5:861–869. https://doi.org/10.1016/S1293-2558(03)00016-5
Essalim R, Ammar A, Zamama M, Mauvy F (2020) A study on structural properties, conductivity and FT-IR spectroscopy of Cu–Al doubly substituted Bi4V2O11. J Solid State Chem 288:121405. https://doi.org/10.1016/j.jssc.2020.121405
Abrahams I, Krok F, Malys M, Wrobel W (2005) Phase transition studies in BIMEVOX solid electrolytes using AC impedance spectroscopy. Solid State Ionics 176:2053–2058. https://doi.org/10.1016/j.ssi.2004.08.044
Pasciak G, Prociow K, Mielcarek W et al (2001) Solid electrolytes for gas sensors and fuel cells applications. J Eur Ceram Soc 21:1867–1870. https://doi.org/10.1016/S0955-2219(01)00132-7
Anwar K, Naqvi FK, Beg S, Haneef S (2023) Photocatalytic degradation of MB dye and paracetamol drug, via hydrothermally synthesised praseodymium doped Bi4V2O11 nanoparticles. J Mol Struct 1272:134183. https://doi.org/10.1016/j.molstruc.2022.134183
Lu Y, Pu Y, Wang J et al (2015) On structure and methylene blue degradation activity of an Aurivillius-type photocatalyst of Bi4V2O11 nanoparticles. Appl Surf Sci 347:719–726. https://doi.org/10.1016/j.apsusc.2015.04.164
Tripathy D, Saikia A, Tado GT, Pandey A (2019) Role of Al and Ti doping in modulating electrical properties of BIVOX system. J Adv Ceram 8:489–499. https://doi.org/10.1007/s40145-019-0329-1
Al-Areqi N, Umair M, Senan A et al (2022) Mesoporous nano-sized BiFeVOx.y phases for removal of organic dyes from wastewaters by visible light photocatalytic degradation. Nanomaterials 12:1383. https://doi.org/10.3390/nano12081383
Trzciński K, Borowska-Centkowska A, Sawczak M, Lisowska-Oleksiak A (2015) Photoelectrochemical properties of BIMEVOX (ME=Cu, Zn, Mn) electrodes in contact with aqueous electrolyte. Solid State Ionics 271:63–68. https://doi.org/10.1016/j.ssi.2014.10.008
Agnaou A, Mhaira W, Essalim R et al (2023) Structural study and ionic conductivity of Bi4V2−xSix/2Px/2O11−δ (0.0 ≤ x ≤ 0.5) compounds. J Solid State Chem 318:123730. https://doi.org/10.1016/j.jssc.2022.123730
Mhaira W, Agnaou A, Essalim R et al (2023) Effect of simultaneous Cu and Nb doping Bi4V2O11 on structural and electrical properties of Bi4V2−xCux/2Nbx/2O11−3x/4. J Solid State Chem 320:123878. https://doi.org/10.1016/j.jssc.2023.123878
Scherrer P (1912) Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. Kolloidchemie Ein Lehrbuch. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 387–409
Agnaou A, Mhaira W, Essalim R et al (2023) Correction: new silicon substituted BiMeVO x : synthesis and study of structural properties in relation to ionic conductivity. RSC Adv 13:8943–8943. https://doi.org/10.1039/D3RA90021E
Alga M, Ammar A, Essalim R et al (2005) Synthesis, sintering and electrical properties of P-doped Bi4V2O11 ceramics. Solid State Sci 7:1173–1179. https://doi.org/10.1016/j.solidstatesciences.2005.06.011
Essalim R, Tanouti B, Bonnet J-P, Réau JM (1992) Elaboration and electrical properties of (0.20 ⩽ x ⩽ 0.55) ceramics with the Y-Bi4V2O11 type structure. Mater Lett 13:382–386. https://doi.org/10.1016/0167-577X(92)90073-S
Essalim R, Ammar A, Tanouti B, Mauvy F (2016) Synthesis, thermal and electrical properties of Al-doped Bi4V1.8Cu0.2O10.7. J Solid State Chem 240:122–125. https://doi.org/10.1016/j.jssc.2016.05.026
Shannon RD (1976) Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A 32:751–767. https://doi.org/10.1107/S0567739476001551
Tripathy D, Saikia A, Tado GT, Pandey A (2018) Dielectric study of Ti-doped Bi2VO5.5 solid electrolyte. Indian J Phys 93:845–859. https://doi.org/10.1007/s12648-018-1356-4
Sharma S, Yashwanth PK, Roy B (2021) Deactivation study of the BICOVOX catalysts used in low temperature steam reforming of ethanol for H2 production. J Phys Chem Solids 156:110138. https://doi.org/10.1016/j.jpcs.2021.110138
Patwe SJ, Patra A, Dey R et al (2013) Probing the local structure and phase transitions of Bi 4 V 2 O 11 -based fast ionic conductors by combined Raman and XRD studies. J Am Ceram Soc 96:3448–3456. https://doi.org/10.1111/jace.12490
Yue Y, Dzięgielewska A, Krok F et al (2022) Local structure and conductivity in the BIGAVOX system. J Phys Chem C 126:2108–2120. https://doi.org/10.1021/acs.jpcc.1c08825
Yue Y, Dzięgielewska A, Hull S et al (2022) Local structure in a tetravalent-substituent BIMEVOX system: BIGEVOX. J Mater Chem A 10:3793–3807. https://doi.org/10.1039/D1TA07547K
Joubert O, Jouanneaux A, Ganne M (1994) Crystal structure of low-temperature form of bismuth vanadium oxide determined by rietveld refinement of X-ray and neutron diffraction data (α - Bi4V2O11). Mater Res Bull 29:175–184. https://doi.org/10.1016/0025-5408(94)90138-4
Hardcastle FD, Wachs IE (1991) Determination of vanadium-oxygen bond distances and bond orders by Raman spectroscopy. J Phys Chem 95:5031–5041. https://doi.org/10.1021/j100166a025
Lazure S, Vannier RN, Nowogrocki G et al (1995) BICOVOX family of oxide anion conductors: chemical, electrical and structural studies. J Mater Chem 5:1395–1403. https://doi.org/10.1039/jm9950501395
Zhao X, Duan Z, Chen L (2019) Bi-quantum-dot-decorated Bi4V2O11 hollow nanocakes: synthesis, characterization, and application as photocatalysts for CO 2 reduction. Ind Eng Chem Res 58:10402–10409. https://doi.org/10.1021/acs.iecr.9b01737
Li J, Lu P, Deng W, et al (2020) Facile synthesis of sheet-like BiVO4/Bi4V2O11 composite for enhanced photocatalytic properties. Mater Chem Phys 123489. https://doi.org/10.1016/j.matchemphys.2020.123489
Liang M, Yang Z, Mei Y et al (2018) Dye-sensitized-assisted, enhanced photocatalytic activity of TiO 2 /Bi 4 V 2 O 11. NANO 13:1850028. https://doi.org/10.1142/S1793292018500285
Al-Areqi NAS, Beg S, Al-Alas A, Hafeez S (2013) Stabilized γ-BIMNVOX solid electrolyte: ethylene glycol–citrate sol–gel synthesis, microwave-assisted calcination, and structural and electrical characterization. J Alloys Compd 581:79–85. https://doi.org/10.1016/j.jallcom.2013.07.038
Buyanova ES, Michaylovkaya ZA, Yurchenko MV, Lipina OA (2020) Photocatalytic characteristics of complex oxides Bi4V1.8Me0.2O11–d (Me = Co, Cu, Fe, Mn, Nb). Russ J Phys Chem A 94:2527–2533. https://doi.org/10.1134/S0036024420120067
Al-Areqi NAS, Al-Kamali ASN, Ghaleb KAS et al (2014) Influence of phase stabilization and perovskite vanadate oxygen vacancies of the BINIVOX catalyst on photocatalytic degradation of azo dye under visible light irradiation. Radiat Eff Defects Solids 169:117–128. https://doi.org/10.1080/10420150.2013.848448
Lin Y, Lu C, Wei C (2019) Microstructure and photocatalytic performance of BiVO4 prepared by hydrothermal method. J Alloys Compd 781:56–63. https://doi.org/10.1016/j.jallcom.2018.12.071
Acknowledgements
The authors are grateful to the Cadi Ayyad University Analysis and Characterization Center (CAC) for providing them with materials characterization techniques.
Author information
Authors and Affiliations
Contributions
A. Agnaou: investigation, writing original draft, formal analysis; W. Mhaira: helped the interpretation of results; R. Essalim: writing review and editing; M. ALGA: investigation; M. Zamama: investigation; F. Mauvy: investigation; A. Ammar: conceived the idea and supervision.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Agnaou, A., Mhaira, W., Essalim, R. et al. Effect of the doping element on the structure and UV–visible properties in the system Bi4V1.7(Si,Me)0.3O11-δ (Me = Si, P, Cu, and Co). Ionics 29, 4923–4932 (2023). https://doi.org/10.1007/s11581-023-05185-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05185-7