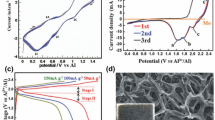
Abstract
Rechargeable aluminum ion batteries (RAIBs) are a very attractive option for large-scale energy storage thanks to their promising theoretical capacity, high energy density, low cost, abundant earth resources, and environmental friendliness. While the cathode materials chosen and prepared are so essential for the electrochemical performance of RAIBs that extensive efforts and research have been done. In this study, the electrochemical performances of RAIBs were optimally improved by the chemical polymerization of triphenylamine to obtain polytriphenylamine (PTPAn) as the cathode material. The polymerization process improved the spatial reticulate structure of triphenylamine, gained a three-dimensional mesh-like nanostructure, which provided more chemical reaction sites and ion reaction channels, greatly increased the specific surface area, and accelerated the electrochemical reaction kinetics. On this basis, a stable discharge-specific capacity of around 137.4 mAh g−1 was achieved at high current densities of 1 A g−1 for the PTPAn cathode, and the Coulombic efficiency was maintained at about 99% after the life of 500 cycles. The understanding and appreciation of the charging and discharging working principle of PTPAn material as RAIBs cathode, meantime, were deepened by a multitude of ex-situ experiments. These findings are anticipated to serve as the cornerstone for the subsequent development of large-scale RAIBs systems for energy storage that use organic polymers as the cathode material.







Similar content being viewed by others
Data availability
Data will be made available upon request.
References
Yang H, Li H, Li J, Sun Z, He K, Cheng HM et al (2019) The rechargeable aluminum battery: opportunities and challenges. Angew Chem Int Ed Engl 58(35):11978–11996
Dunn B, Kamath H, Tarascon JM (2011) Electrical energy storage for the grid: a battery of choices. Science 334(6058):928–935
Chu S, Cui Y, Liu N (2016) The path towards sustainable energy. Nat Mater 16(1):16
Hu Y, Sun D, Luo B, Wang L (2019) Recent progress and future trends of aluminum batteries. Energy Technol 7(1):86–106
Elia GA, Marquardt K, Hoeppner K, Fantini S, Lin R, Knipping E et al (2016) An overview and future perspectives of aluminum batteries. Adv Mater 28(35):7564–7579
Lin M-C, Gong M, Lu B, Wu Y, Wang D-Y, Guan M et al (2015) An ultrafast rechargeable aluminium-ion battery. Nature 520(7547):324–328
Zhou Q, Zheng Y, Wang D, Lian Y, Ban C, Zhao J et al (2020) Cathode materials in non-aqueous aluminum-ion batteries: progress and challenges. Ceram Int 46(17):26454–26465
Zhang K, Kirlikovali KO, Suh JM, Choi J-W, Jang HW, Varma RS et al (2020) Recent advances in rechargeable aluminum-ion batteries and considerations for their future progress. ACS Appl Energy Mater 3(7):6019–6035
Wang H, Bai Y, Chen S, Luo X, Wu C, Wu F et al (2015) Binder-Free V2O5 Cathode for greener rechargeable aluminum battery. ACS Appl Mater Interfaces 7(1):80–84
Wang S, Jiao S, Wang J, Chen HS, Tian D, Lei H et al (2016) High-performance aluminum-ion battery with CuS@C microsphere composite cathode. ACS Nano 11(1):469–477
Nacimiento F, Cabello M, Alcántara R, Pérez-Vicente C, Lavela P, Tirado JL (2018) Exploring an aluminum ion battery based on molybdite as working electrode and ionic liquid as electrolyte. J Electrochem Soc 165(13):A2994–A29A9
Qiao J, Zhou H, Liu Z, Wen H, Yang J (2019) Defect-free soft carbon as cathode material for Al-ion batteries. Ionics 25(3):1235–1242
Qiao J, Zhou H, Liu Z, Wen H, Du J, Wei G et al (2020) Dense integration of graphene paper positive electrode materials for aluminum-ion battery. Ionics 26(1):245–254
Meng P, Huang J, Yang Z, Wang F, Lv T, Zhang J et al (2022) A low-cost and air-stable rechargeable aluminum-ion battery. Adv Mater 34(8):2106511
Wang S, Huang S, Yao M, Zhang Y, Niu Z (2020) Engineering active sites of polyaniline for AlCl2 (+) storage in an aluminum-ion battery. Angew Chem Int Ed Engl 59(29):11800–11807
Wang D, Hu H, Liao Y, Kong D, Cai T, Gao X et al (2020) High-performance aluminum-polyaniline battery based on the interaction between aluminum ion and -NH groups. Sci China Mater 64(2):318–328
Liao Y, Wang D, Li X, Tian S, Hu H, Kong D et al (2020) High performance aluminum ion battery using polyaniline/ordered mesoporous carbon composite. J Power Sources 477:228702
Meng J, Zhu L, Haruna AB, Ozoemena KI, Pang Q (2021) Charge storage mechanisms of cathode materials in rechargeable aluminum batteries. Sci China Chem 64(11):1888–1907
Zhang H, Huang L, Xu H, Zhang X, Chen Z, Gao C et al (2022) A polymer electrolyte with a thermally induced interfacial ion-blocking function enables safety-enhanced lithium metal batteries. eScience 2:201–208
Mu P, Zhang H, Jiang H, Dong T, Zhang S, Wang C et al (2021) Bioinspired antiaging binder additive addressing the challenge of chemical degradation of electrolyte at cathode/electrolyte interphase. J Am Chem Soc 43:143
Wang C, Ma Y, Du X, Zhang H, Xu G, Cui G (2022) A polysulfide radical anions scavenging binder achieves long-life lithium–sulfur batteries. Battery Energy 1(3):20220010
Jiang M, Mu P, Zhang H, Dong T, Tang B, Qiu H et al (2022) An endotenon sheath-inspired double-network binder enables superior cycling performance of silicon electrodes. Nano-Micro Lett 14:87. https://doi.org/10.1007/s40820-022-00833-5
Peng Z, Yi X, Liu Z, Shang J, Wang D (2016) Triphenylamine-based metal–organic frameworks as cathode materials in lithium-ion batteries with coexistence of redox active sites, high working voltage, and high rate stability. ACS Appl Mater Interfaces 8(23):14578–14585
Yamamoto K, Suemasa D, Masuda K, Aita K, Endo T (2018) Hyperbranched triphenylamine polymer for ultrafast battery cathode. ACS Appl Mater Interfaces 10(7):6346–6353
Lian X, Zhao Z, Cheng D (2017) Recent progress on triphenylamine materials: synthesis, properties, and applications. Mol Cryst Liq Cryst 648(1):223–235
Chen Z, Su C, Zhu X, Xu R, Xu L, Zhang C (2018) Micro-/mesoporous conjugated polymer based on star-shaped triazine-functional triphenylamine framework as the performance-improved cathode of li-organic battery. J Polym Sci A Polym Chem 56(22):2574–2583
Mo L, Zhou G, Ge P, Miao Y-E, Liu T (2022) Flexible polytriphenylamine-based cathodes with reinforced energy-storage capacity for high-performance sodium-ion batteries. Sci China Mater 65(1):32–42
Ni W, Cheng J, Li X, Gu G, Huang L, Guan Q et al (2015) Polymeric cathode materials of electroactive conducting poly (triphenylamine) with optimized structures for potential organic pseudo-capacitors with higher cut-off voltage and energy density. RSC Adv 5(12):9221–9227
Li X, Zhang Y, Xing W, Li L, Xue Q, Yan Z (2016) Sandwich-like graphene/polypyrrole/layered double hydroxide nanowires for high-performance supercapacitors. J Power Sources 331:67–75
Pu X, Zhao D, Fu C, Chen Z, Cao S, Wang C et al (2021) Understanding and calibration of charge storage mechanism in cyclic voltammetry curves. Angew Chem 133(39):21480–21488
Acknowledgements
The authors thank the support from the Institute of Green Materials and Metallurgy, School of Materials Science and Engineering and Cranfield Tech Futures Graduate Institute of Jiangsu University, the Youth Fund project of Natural Science Foundation of Jiangsu Province, and the School of Water, Energy and Environment of Cranfield University.
Funding
This work was supported by the Institute of Green Materials and Metallurgy, Jiangsu University (No. 4023000047, 4023000048), the Youth Fund project of Natural Science Foundation of Jiangsu Province (No. BK20200898), and the Incubation Fund of School of Materials Science and Engineering, Jiangsu University, Scientific Research and Technology Development Project of Hezhou (No. HEKEJI20012).
Author information
Authors and Affiliations
Contributions
FT did the investigation and prepared Figs. 1–9.
GW did the data curation and wrote the main manuscript text.
XX and WX (Weize Xu) did the formal analysis and resources parts.
WX (Wei Xie), ZL, JY, XL, and JQ did the supervision and writing – reviewing and editing.
All authors reviewed the manuscript.
All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The authors declare that this declaration is “not applicable.”
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 235 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tao, F., Wei, G., Xu, X. et al. Spatial reticulate polytriphenylamine cathode material with enhanced capacity for rechargeable aluminum ion batteries. Ionics 29, 3619–3627 (2023). https://doi.org/10.1007/s11581-023-05096-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05096-7