Abstract
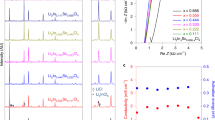
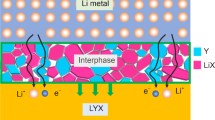
Chloride solid-state electrolytes (SSEs) are of great interest because of their wide electrochemical window. However, most chloride SSEs have lower ionic conductivity, which makes it difficult to apply chloride SSEs in high-rate all-solid-state lithium-ion batteries (ASSLIBs). Therefore, in this study, Li3HoCl6 was synthesized by a wet chemical method, followed by Br substitution (Li3HoCl6-xBrx) to improve the ionic conductivity. We found that the crystal structure of the Br substituted product did not change with increasing x to 1 and 2, but its ionic conductivity improved significantly, reaching 1.24 mS cm-1 at 298 K for the Li3HoCl4Br2, which is about ten times higher than that of the unsubstituted product. However, when the amount of substitution is further increased (x = 3), the crystal structure is changed. The present work illustrates that Br substituted Li3HoCl6 is a promising SSE and lays the foundation for subsequent works.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Zhou D, Shanmukaraj D, Tkacheva A, Armand M, Wang G (2019) Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 5:2326. https://doi.org/10.1016/j.chempr.2019.05.009
Wan J, Xie J, Kong X, Liu Z, Liu K, Shi F, Pei A, Chen H, Chen W, Chen J, Zhang X, Zong L, Wang J, Chen LQ, Qin J, Cui Y (2019) Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat Nanotechnol 14:705. https://doi.org/10.1038/s41565-019-0465-3
Asano T, Sakai A, Ouchi S, Sakaida M, Miyazaki A, Hasegawa S (2018) Solid Halide Electrolytes with High Lithium-Ion Conductivity for Application in 4 V Class Bulk-Type All-Solid-State Batteries. Adv Mater 30:e1803075. https://doi.org/10.1002/adma.201803075
Guo Z, Pang Y, Xia S, Xu F, Yang J, Sun L, Zheng S (2021) Uniform and Anisotropic Solid Electrolyte Membrane Enables Superior Solid-State Li Metal Batteries. Adv Sci 8:e2100899. https://doi.org/10.1002/advs.202100899
Wu Z, Xie Z, Yoshida A, Wang Z, Hao X, Abudula A, Guan G (2019) Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review. Renew Sustain Energy Rev 109:367. https://doi.org/10.1016/j.rser.2019.04.035
Yang S, Takahashi M, Yamamoto K, Ohara K, Watanabe T, Uchiyama T, Takami T, Sakuda A, Hayashi A, Tatsumisago M, Uchimoto Y (2022) Studies on the inhibition of lithium dendrite formation in sulfide solid electrolytes doped with LiX (X = Br, I). Solid State Ion 377:115869. https://doi.org/10.1016/j.ssi.2022.115869
Zhang Y, Sun C (2021) Composite Lithium Protective Layer Formed In Situ for Stable Lithium Metal Batteries. ACS Appl Mater Interfaces 13:12099. https://doi.org/10.1021/acsami.1c00745
Kato Y, Kawamoto K, Kanno R, Hirayama M (2012) Discharge Performance of All-Solid-State Battery Using a Lithium Superionic Conductor Li10GeP2S12. Electrochemistry 80:749. https://doi.org/10.5796/electrochemistry.80.749
Lee JS, Park YJ (2021) Comparison of LiTaO3 and LiNbO3 Surface Layers Prepared by Post-and Precursor-Based Coating Methods for Ni-Rich Cathodes of All-Solid-State Batteries. ACS Appl Mater Interfaces 13:38333. https://doi.org/10.1021/acsami.1c10294
Li Z, Wang Z, Miao Y, Ma Y, Zhang H, Shi X, Song D, Zhang L, Zhu L (2022) Constructing rapid ionic transfer layer to boost the performance of LiCoO2 cathode with high mass loading for all-solid-state lithium battery. J Power Sources 541:231703. https://doi.org/10.1016/j.jpowsour.2022.231703
Liu Z, Fu W, Payzant EA, Yu X, Wu Z, Dudney NJ, Kiggans J, Hong K, Rondinone AJ, Liang C (2013) Anomalous high ionic conductivity of nanoporous beta-Li3PS4. J Am Chem Soc 135:975. https://doi.org/10.1021/ja3110895
Morino Y (2022) Degradation rate at the Solid–Solid interface of sulfide-based solid Electrolyte–Cathode active material. J Power Sources 541:231672. https://doi.org/10.1016/j.jpowsour.2022.231672
Tsukasaki H, Igarashi K, Wakui A, Yaguchi T, Nakajima H, Kimura T, Sakuda A, Tatsumisago M, Hayashi A, Mori S (2021) In situ observation of the deterioration process of sulfide-based solid electrolytes using airtight and air-flow TEM systems. Microscopy 70:519. https://doi.org/10.1093/jmicro/dfab022
Jiang Z, Wang S, Chen X, Yang W, Yao X, Hu X, Han Q, Wang H (2020) Tape-Casting Li0.34La0.56TiO3 Ceramic Electrolyte Films Permit High Energy Density of Lithium-Metal Batteries. Adv Mater 32:e1906221. https://doi.org/10.1002/adma.201906221
Kim M, Kim G, Lee H (2021) Tri-Doping of Sol-Gel Synthesized Garnet-Type Oxide Solid-State Electrolyte. Micromachines 12:134. https://doi.org/10.3390/mi12020134
Liu Q, Geng Z, Han C, Fu Y, Li S, He Y-B, Kang F, Li B (2018) Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. J Power Sources 389:120. https://doi.org/10.1016/j.jpowsour.2018.04.019
Wu N, Chien P-H, Qian Y, Li Y, Xu H, Grundish NS, Xu B, Jin H, Hu Y-Y, Yu G, Goodenough JB (2020) Enhanced Surface Interactions Enable Fast Li+ Conduction in Oxide/Polymer Composite Electrolyte. Angew Chem 59:4131. https://doi.org/10.1002/anie.201914478
Kim SY, Kaup K, Park K-H, Assoud A, Zhou L, Liu J, Wu X, Nazar LF (2021) Lithium Ytterbium-Based Halide Solid Electrolytes for High Voltage All-Solid-State Batteries. ACS Mater Lett 3:930. https://doi.org/10.1021/acsmaterialslett.1c00142
Li X, Liang J, Chen N, Luo J, Adair KR, Wang C, Banis MN, Sham TK, Zhang L, Zhao S, Lu S, Huang H, Li R, Sun X (2019) Water-Mediated Synthesis of a Superionic Halide Solid Electrolyte. Angew Chem 58:16427. https://doi.org/10.1002/anie.201909805
Bohnsack A, Stenzel F, Zajonc A, Balzer G, Wickleder MS, Meyer G (1997) Ternary Halides of the A3MX6 Type. VI. Ternary Chlorides of the Rare-Earth Elements with Lithium, Li3MCl6 (M = Tb-Lu, Y, Sc): Synthesis, Crystal Structures, and Ionic Motion. Z Anorg Allg Chem 623:1067. https://doi.org/10.1002/zaac.19976230710
Li X, Liang J, Luo J, Norouzi Banis M, Wang C, Li W, Deng S, Yu C, Zhao F, Hu Y, Sham T-K, Zhang L, Zhao S, Lu S, Huang H, Li R, Adair KR, Sun X (2019) Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries. Energ Environ Sci 12:2665. https://doi.org/10.1039/c9ee02311a
Liang J, Li X, Wang S, Adair KR, Li W, Zhao Y, Wang C, Hu Y, Zhang L, Zhao S, Lu S, Huang H, Li R, Mo Y, Sun X (2020) Site-Occupation-Tuned Superionic LixScCl3+xHalide Solid Electrolytes for All-Solid-State Batteries. J Am Chem Soc 142:7012. https://doi.org/10.1021/jacs.0c00134
Wang K, Ren Q, Gu Z, Duan C, Wang J, Zhu F, Fu Y, Hao J, Zhu J, He L, Wang CW, Lu Y, Ma J, Ma C (2021) A cost-effective and humidity-tolerant chloride solid electrolyte for lithium batteries. Nat Commun 12:4410. https://doi.org/10.1038/s41467-021-24697-2
Liu Z, Ma S, Liu J, Xiong S, Ma Y, Chen H (2020) High Ionic Conductivity Achieved in Li3Y(Br3Cl3) Mixed Halide Solid Electrolyte via Promoted Diffusion Pathways and Enhanced Grain Boundary. ACS Energy Lett 6:298. https://doi.org/10.1021/acsenergylett.0c01690
Wang C, Liang J, Luo J, Liu J, Li X, Zhao F, Li R, Huang H, Zhao S, Zhang L, Wang J, Sun X (2021) A universal wet-chemistry synthesis of solid-state halide electrolytes for all-solid-state lithium-metal batteries. Sci Adv 7:1896. https://doi.org/10.1126/sciadv.abh1896
Liang J, Maas E, Luo J, Li X, Chen N, Adair KR, Li W, Li J, Hu Y, Liu J, Zhang L, Zhao S, Lu S, Wang J, Huang H, Zhao W, Parnell S, Smith RI, Ganapathy S et al (2022) A Series of Ternary Metal Chloride Superionic Conductors for High-Performance All-Solid-State Lithium Batteries. Adv Energy Mater 12:2103921. https://doi.org/10.1002/aenm.202103921
Li X, Liang J, Kim JT, Fu J, Duan H, Chen N, Li R, Zhao S, Wang J, Huang H, Sun X (2022) Highly Stable Halide-Electrolyte-Based All-Solid-State Li-Se Batteries. Adv Mater 34:e2200856. https://doi.org/10.1002/adma.202200856
Hu Y, Li Z, Hu Z, Wang L, Ma R, Wang J (2020) Engineering Hierarchical CoO Nanospheres Wrapped by Graphene via Controllable Sulfur Doping for Superior Li Ion Storage. Small 16:e2003643. https://doi.org/10.1002/smll.202003643
Shi X, Zeng Z, Zhang H, Huang B, Sun M, Wong HH, Lu Q, Luo W, Huang Y, Du Y, Yan CH (2021) Gram-Scale Synthesis of Nanosized Li3HoBr6 Solid Electrolyte for All-Solid-State Li-Se Battery. Small Methods 5:e2101002. https://doi.org/10.1002/smtd.202101002
Luo X, Wu X, Xiang J, Cai D, Li M, Wang X, Xia X, Gu C, Tu J (2021) Heterovalent Cation Substitution to Enhance the Ionic Conductivity of Halide Electrolytes. ACS Appl Mater Interfaces 13:47610. https://doi.org/10.1021/acsami.1c13295
Shao Q, Yan C, Gao M, Du W, Chen J, Yang Y, Gan J, Wu Z, Sun W, Jiang Y, Liu Y, Gao M, Pan H (2022) New Insights into the Effects of Zr Substitution and Carbon Additive on Li3-xEr1-xZrxCl6 Halide Solid Electrolytes. ACS Appl Mater Interfaces 14:8095. https://doi.org/10.1021/acsami.1c25087
Shi X, Zeng Z, Sun M, Huang B, Zhang H, Luo W, Huang Y, Du Y, Yan C (2021) Fast Li-ion Conductor of Li3HoBr6 for Stable All-Solid-State Lithium-Sulfur Battery. Nano Lett 21:9325. https://doi.org/10.1021/acs.nanolett.1c03573
Kimura T, Inoue A, Nagao K, Inaoka T, Kowada H, Sakuda A, Tatsumisago M, A, Hayashi. (2022) Characteristics of a Li3BS3 Thioborate Glass Electrolyte Obtained via a Mechanochemical Process. ACS Appl Energy Mater 5:1421. https://doi.org/10.1021/acsaem.1c02452
Zeng Y, Mao P-L, Jiang SP, Wu P, Zhang L, Wu P (2011) Prediction of oxygen ion conduction from relative Coulomb electronic interactions in oxyapatites. J Power Sources 196:4524. https://doi.org/10.1016/j.jpowsour.2011.01.017
Li X, Liang J, Adair KR, Li J, Li W, Zhao F, Hu Y, Sham TK, Zhang L, Zhao S, Lu S, Huang H, Li R, Chen N, Sun X (2020) Origin of Superionic Li3Y1-xInxCl6 Halide Solid Electrolytes with High Humidity Tolerance. Nano Lett 20:4384. https://doi.org/10.1021/acs.nanolett.0c01156
Han F, Westover AS, Yue J, Fan X, Wang F, Chi M, Leonard DN, Dudney NJ, Wang H, Wang C (2019) High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat Energy 4:187. https://doi.org/10.1038/s41560-018-0312-z
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 52261040, 51971104).
Author information
Authors and Affiliations
Contributions
Jun Peng: Investigation, Data Curation, Writing - Original Draft; Lei Xian: Data Curation, Supervision; Ling-Bin Kong: Conceptualization, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, J., Xian, L. & Kong, LB. Effects of Br substitution to improve the ionic conductivity of chloride electrolytes without changing crystal structure. Ionics 29, 2657–2664 (2023). https://doi.org/10.1007/s11581-023-05028-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05028-5