Abstract
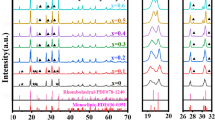
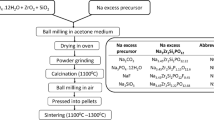
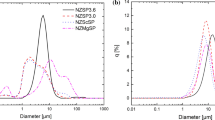
NASICON-like Na3Zr2(SiO4)2PO4 (NZSP) ceramic solid electrolyte with high ionic conductivity, safety, and durability becomes the main focus and attention as an alternative for traditional liquid electrolytes. NZSP containing NH4H2PO4 and Na3PO4⋅12H2O as the phosphate source has been extensively studied as a solid electrolyte, but a deep understanding of the relationship between crystal growth and ionic conductivity is still lacking. Herein, we synthesized NZSP via a solid-state reaction using NaH2PO4 as the phosphate source. The impact of different sintering holding times on the crystal phase, microstructure, ionic conductivity, and relaxation time of NZSP solid electrolytes was investigated. Microstructure studies revealed that the faceted NZSP sintered at 1100 °C for 24 h has the lowest formation of ZrO2 and highest densification with the least pores. In addition, the sample achieved the highest room temperature ionic conductivity (4.11 ⨯ 10−4 S cm−1) and the shortest relaxation time (0.4 μs), which are also crucial factors for the development of rechargeable all-solid-state batteries (ASSBs).





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Feng X, Ouyang M, Liu X et al (2018) Thermal runaway mechanism of lithium ion battery for electric vehicles: a review. Energy Storage Mater 10:246–267. https://doi.org/10.1016/j.ensm.2017.05.013
Chong MK, Zainuddin Z, Hj Jumali MH, Omar FS (2022) Effects of excess Na and P on ionic conductivity of Na3Zr2(SiO4)2PO4. In: The 12th International Fundamental Science Congress 2021 (iFSC2021): “Engaging Fundamental Science in the New Norm.” Jabatan Fizik, Fakulti Sains, Universiti Putra Malaysia, Serdang, pp 84–87
Zheng F, Kotobuki M, Song S et al (2018) Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources 389:198–213. https://doi.org/10.1016/j.jpowsour.2018.04.022
Biemolt J, Jungbacker P, Van TT et al (2020) Beyond lithium-based batteries. Materials (Basel) 13:425. https://doi.org/10.3390/ma13020425
Majid MF, Zaid HFM, Kait CF et al (2022) Ionic liquid@metal-organic framework as a solid electrolyte in a lithium-ion battery: current performance and perspective at molecular level. Nanomaterials 12:1076. https://doi.org/10.3390/nano12071076
Nayak PK, Yang L, Brehm W, Adelhelm P (2018) From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew Chemie - Int Ed 57:102–120. https://doi.org/10.1002/anie.201703772
Kit CM, Zainuddin Z, Jumali MH (2020) NaH2PO4 As raw material for phosphate in Na3Zr2(SiO4)2PO4 NASICON-type ceramic solid electrolyte. In: Graduate Colloquium Research Book of the Department of Applied Physics 2020. Fakulti Sains dan Teknologi, Universiti Kebangsaan Malaysia, Bangi, pp 31–34
Lu X, Xia G, Lemmon JP, Yang Z (2010) Advanced materials for sodium-beta alumina batteries: status, challenges and perspectives. J Power Sources 195:2431–2442. https://doi.org/10.1016/j.jpowsour.2009.11.120
Yang FZT, Peterson VK, Schmid S (2021) Composition and temperature dependent structural investigation of the perovskite-type sodium-ion solid electrolyte series Na1/2−xLa1/2−xSr2xZrO3. J Alloys Compd 863:158500. https://doi.org/10.1016/j.jallcom.2020.158500
Goodenough JB, Hong HY-P, Kafalas JA (1976) Fast Na+-ion transport in skeleton structures. Mater Res Bull 11:203–220. https://doi.org/10.1016/0025-5408(76)90077-5
Hong HYP (1978) Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors. Mater Res Bull 13:117–124. https://doi.org/10.1016/0025-5408(78)90075-2
Li S, Xu X, Yao Z et al (2018) A comparative study on the Li+/Na+ transportation in NASICON-type electrolytes. J Phys Chem C 122:20565–20570. https://doi.org/10.1021/acs.jpcc.8b01987
Norhaniza R, Subban RH, Mohamed N, Ahmad A (2012) Chromium substituted LiSn2P3O12 solid electrolyte. Int J Electrochem Sci 7:10254–10265
Park H, Kang M, Park YC et al (2018) Improving ionic conductivity of Nasicon (Na3Zr2Si2PO12) at intermediate temperatures by modifying phase transition behavior. J Power Sources 399:329–336. https://doi.org/10.1016/j.jpowsour.2018.07.113
Ruan Y, Guo F, Liu J et al (2019) Optimization of Na3Zr2Si2PO12 ceramic electrolyte and interface for high performance solid-state sodium battery. Ceram Int 45:1770–1776. https://doi.org/10.1016/j.ceramint.2018.10.062
Khakpour Z (2016) Influence of M: Ce4+, Gd3+ and Yb3+ substituted Na3+xZr2-xMxSi2PO12 solid NASICON electrolytes on sintering, microstructure and conductivity. Electrochim Acta 196:337–347. https://doi.org/10.1016/j.electacta.2016.02.199
Yang J, Wan HL, Zhang ZH et al (2018) NASICON-structured Na3.1Zr1.95Mg0.05Si2PO12 solid electrolyte for solid-state sodium batteries. Rare Met 37:480–487. https://doi.org/10.1007/s12598-018-1020-3
Chen D, Luo F, Zhou W, Zhu D (2018) Influence of Nb5+, Ti4+, Y3+ and Zn2+ doped Na3Zr2Si2PO12 solid electrolyte on its conductivity. J Alloys Compd 757:348–355. https://doi.org/10.1016/j.jallcom.2018.05.116
Narayanan S, Reid S, Butler S, Thangadurai V (2019) Sintering temperature, excess sodium, and phosphorous dependencies on morphology and ionic conductivity of NASICON Na3Zr2Si2PO12. Solid State Ionics 331:22–29. https://doi.org/10.1016/j.ssi.2018.12.003
Chong MK, Zainuddin Z, Omar FS, Jumali MHH (2022) Na3Zr2(SiO4)2PO4 NASICON-type solid electrolyte: influence of milling duration on microstructure and ionic conductivity mechanism. Ceram Int 48:22147–22154. https://doi.org/10.1016/j.ceramint.2022.04.202
Jha PK, Pandey OP, Singh K (2017) Optimization of high conducting Na3Zr2Si2PO12 phase by new phosphate salt for solid electrolyte. SILICON 9:411–419. https://doi.org/10.1007/s12633-015-9396-2
Sun F, Xiang Y, Sun Q et al (2021) Insight into ion diffusion dynamics/mechanisms and electronic structure of highly conductive sodium-rich Na3+xLaxZr2-xSi2PO12 (0 ≤ x ≤ 0.5) solid-state electrolytes. ACS Appl Mater Interfaces 13:13132–13138. https://doi.org/10.1021/acsami.0c21882
Yongzheng J, Tsuyoshi H, Takayuki K (2021) Synthesis and Na+ ion conductivity of stoichiometric Na3Zr2Si2PO12 by liquid-phase sintering with NaPO3 glass. Materials (Basel) 14:3790. https://doi.org/10.3390/ma14143790
Park H, Jung K, Nezafati M et al (2016) Sodium ion diffusion in Nasicon (Na3Zr2Si2PO12) solid electrolytes: effects of excess sodium. ACS Appl Mater Interfaces 8:27814–27824. https://doi.org/10.1021/acsami.6b09992
Bregiroux D, Audubert F, Bernache-Assollant D (2009) Densification and grain growth during solid state sintering of LaPO4. Ceram Int 35:1115–1120. https://doi.org/10.1016/j.ceramint.2008.05.005
Tanaka S (2019) Solid state reactions and sintering. In: Hojo J (ed) Materials Chemistry of Ceramics. Springer Singapore, pp 45–74. https://doi.org/10.1007/978-981-13-9935-0_3
Djohari H, Derby JJ (2009) Transport mechanisms and densification during sintering: II. Grain boundaries. Chem Eng Sci 64:3810–3816. https://doi.org/10.1016/j.ces.2009.05.022
Carter CB, Norton MG (2007) Sintering and grain growth. Ceramic Materials. Springer, New York, pp 427–443
Waetzig K, Rost A, Langklotz U et al (2016) An explanation of the microcrack formation in Li1.3Al0.3Ti1.7(PO4)3 ceramics. J Eur Ceram Soc 36:1995–2001. https://doi.org/10.1016/j.jeurceramsoc.2016.02.042
Yang G, Zhai Y, Yao J et al (2021) A facile method for the synthesis of a sintering dense nano-grained Na3Zr2Si2PO12 Na+-ion solid-state electrolyte. Chem Commun 57:4023–4026. https://doi.org/10.1039/d0cc07261c
Zhai Y, Hou W, Tao M et al (2022) Enabling high-voltage “superconcentrated ionogel-in-ceramic” hybrid electrolyte with ultrahigh ionic conductivity and single Li+-Ion transference number. Adv Mater 34:2205560. https://doi.org/10.1002/adma.202205560
Jonscher AK (1999) Dielectric relaxation in solids. J Phys D Appl Phys 32:R57–R70. https://doi.org/10.1088/0022-3727/32/14/201
Aziz SB, Abdullah RM, Rasheed MA, Ahmed HM (2017) Role of ion dissociation on DC conductivity and silver nanoparticle formation in PVA:AgNt based polymer electrolytes: deep insights to ion transport mechanism. Polymers (Basel) 9:338. https://doi.org/10.3390/polym9080338
Almond DP, West AR (1983) Mobile ion concentrations in solid electrolytes from an analysis of a.c. conductivity. Solid State Ionics 9–10:277–282. https://doi.org/10.1016/0167-2738(83)90247-3
Petrova NR, Noriaki N, Snejana B et al (2011) Temperature-induced phase transformations of the small-pore zirconosilicate Na2ZrSi2O7·H2O. Solid State Sci 13:1187–1190. https://doi.org/10.1016/j.solidstatesciences.2010.06.018
Rusdi H, Mohamed NS, Subban RHY, Rusdi R (2020) Enhancement of electrical properties of NASICON-type solid electrolytes (LiSn2P3O12) via aluminium substitution. J Sci Adv Mater Devices 5:368–377. https://doi.org/10.1016/j.jsamd.2020.06.003
Abdullah AM, Aziz SB, Saeed SR (2021) Structural and electrical properties of polyvinyl alcohol (PVA):methyl cellulose (MC) based solid polymer blend electrolytes inserted with sodium iodide (NaI) salt. Arab J Chem 14:103388. https://doi.org/10.1016/j.arabjc.2021.103388
Lu X, Wang R, Zhang F, Li J (2020) The influence of phosphorous source on the properties of NASICON lithium-ion conductor Li1.3Al0.3Ti1.7(PO4)3. Solid State Ionics 354:115417. https://doi.org/10.1016/j.ssi.2020.115417
Acknowledgements
The authors would like to thank the Department of Applied Physics and Centre for Research and Instrumentation Management (CRIM), Universiti Kebangsaan Malaysia for providing the facilities.
Funding
This work has been supported by the Fundamental Research Grant Scheme (FRGS/1/2017/STG02/UKM/02/4) from the Ministry of Higher Education, Malaysia and also the financial assistance (GP-2019-K007748, GP-2020-K007748) from the Universiti Kebangsaan Malaysia.
Author information
Authors and Affiliations
Contributions
ZZ, FSO, and MHHJ conceived the idea and designed the research. MKC prepared the samples and did all the characterization. The first draft of the manuscript was written by MKC, and all authors reviewed the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chong, M.K., Zainuddin, Z., Omar, F.S. et al. Tuning the growth of faceted Na3Zr2(SiO4)2PO4 NASICON-like solid electrolyte and its effect on microstructure-conductivity relationship. Ionics 29, 3109–3117 (2023). https://doi.org/10.1007/s11581-023-05014-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05014-x