Abstract
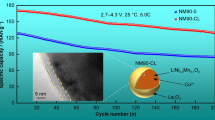
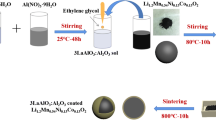
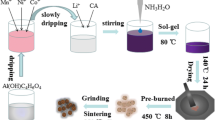
Among various cathode materials for lithium-ion batteries, xLi2MnO3⋅(1-x)LiMO2(M=Ni, Co, Mn) with layered structure has great potential due to its high specific capacity. In this work, LaPO4-coated Li1.2Mn0.54Co0.13Ni0.13O2 cathode material was successfully synthesized by wet chemical deposition method, and La doping was achieved by calcination. The results of SEM, TEM, and HRTEM showed that LaPO4 is successfully coated on the surface of the material, La is successfully doped into the material, and the interlayer spacing of the material becomes larger after modification. The results of XRD and XPS also showed that La was successfully doped into the material. The electrochemical characterization results showed that LaPO4 modification significantly improved the electrochemical performance of the material. Most importantly, the lithium-ion diffusion coefficient of 2 wt%-LaPO4 is as high as 4.07 × 10−14 cm2·s−1, which is four times that of the pristine material. Its specific capacity at 10 C is 100.3 mAh·g−1, which is about 90% higher than that of the unmodified material 52.8 mAh·g−1. The LaPO4-modified lithium-rich manganese-based cathode material has such good rate performance, which is attributed to the triple effect of LaPO4 modification on promoting lithium-ion diffusion, which promotes the diffusion of lithium ion in three stages, from bulk phase to interface and then to electrolyte. The schematic can be seen in the graphical abstract.
Graphical abstract

LaPO4 coating and La3+ doping schematic diagram










Similar content being viewed by others
References
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Ritchie A, Howard W (2006) Recent developments and likely advances in lithium-ion batteries. J Power Sources 162:809–812
Myung S-T, Maglia F, Park K-J, Yoon CS, Lamp P, Kim S-J, Sun Y-K (2017) Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. Acs Energy Letters 2:196–223
Larcher D, Tarascon JM (2015) Towards greener and more sustainable batteries for electrical energy storage. Nat Chem 7:19–29
Zhang L, Wang H, Wang L, Fang H, Li X, Gao H, Zhang A, Song Y (2016) High electrochemical performance of lithium-rich Li1.2Mn0.54NixCoyO2 cathode materials for lithium-ion batteries. Mater Lett 185:100–103
Stephan AK (2019) The age of Li-ion batteries. Joule 3:2583–2584
Armand M, Tarascon JM (2008) Building better batteries. Nature 451:652–657
Grey CP, Tarascon JM (2017) Sustainability and in situ monitoring in battery development. Nat Mater 16:45–56
Sathiya M, Abakumov AM, Foix D, Rousse G, Ramesha K, Saubanere M, Doublet ML, Vezin H, Laisa CP, Prakash AS, Gonbeau D, VanTendeloo G, Tarascon JM (2015) Origin of voltage decay in high-capacity layered oxide electrodes. Nat Mater 14:230–238
Yu H, Zhou H (2013) High-energy cathode materials (Li2MnO3-LiMO2) for lithium-ion batteries. J Phys Chem 4:1268–1280
Ding X, Li Y-x, Deng M-m, Wang S, Aqsa Y, Hu Q, Chen C-h (2019) Cesium doping to improve the electrochemical performance of layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode material. J Alloys Compd 791:100–108
Rao RR, Tulodziecki M, Han B, Risch M, Abakumov A, Yu Y, Karayaylali P, Gauthier M, Escudero-Escribano M, Orikasa Y, Shao-Horn Y (2021) Reactivity with water and bulk ruthenium redox of lithium ruthenate in basic solutions. Adv Funct Mater 31:2002249
Boulineau A, Simonin L, Colin J-F, Bourbon C, Patoux S (2013) First evidence of manganese-nickel segregation and densification upon cycling in Li-rich layered oxides for lithium batteries. Nano Lett 13:3857–3863
Li W, Liu X, Celio H, Smith P, Dolocan A, Chi M, Manthiram A (2018) Mn versus Al in layered oxide cathodes in lithium-ion batteries: a comprehensive evaluation on long-term cyclability. Adv Energy Mater 8:1704154
Singer A, Zhang M, Hy S, Cela D, Fang C, Wynn TA, Qiu B, Xia Y, Liu Z, Ulvestad A, Hua N, Wingert J, Liu H, Sprung M, Zozulya AV, Maxey E, Harder R, Meng YS, Shpyrko OG (2018) Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging. Nat Energy 3:641–647
Ji X, Xia Q, Xu Y, Feng H, Wang P, Tan Q (2021) A review on progress of lithium-rich manganese-based cathodes for lithium ion batteries. J Power Sources 487:229362
Xiao R, Li H, Chen L (2012) Density functional investigation on Li2MnO3. Chem Mater 24:4242–4251
Vetter J, Novak P, Wagner MR, Veit C, Moller KC, Besenhard JO, Winter M, Wohlfahrt-Mehrens M, Vogler C, Hammouche A (2005) Ageing mechanisms in lithium-ion batteries. J Power Sources 147:269–281
Aurbach D (2000) Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J Power Sources 89:206–218
Assat G, Delacourt C, Dalla Corte DA, Tarascon J-M (2016) Practical assessment of anionic redox in Li-rich layered oxide cathodes: a mixed blessing for high energy Li-ion batteries. J Electrochem Soc 163:A2965–A2976
Wang A, Kadam S, Li H, Shi S, Qi Y (2018) Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries. Npj Comput Mater 4:15
Qing R-P, Shi J-L, Xiao D-D, Zhang X-D, Yin Y-X, Zhai Y-B, Gu L, Guo Y-G (2016) Enhancing the kinetics of Li-rich cathode materials through the pinning effects of gradient surface Na+ doping. Adv Energy Mater 6:1501914
Niu B, Li J, Liu Y, Li Z, Yang Z (2019) Re-understanding the function mechanism of surface coating: modified Li-rich layered Li1.2Mn0.54Ni0.13Co0.13O2 cathodes with YF3 for high performance lithium-ions batteries. Ceram Int 45:12484–12494
Shu W, Jian Z, Zhou J, Zheng Y, Chen W (2021) Boosting the electrochemical performance of Li1.2Ni0.13Co0.13Mn0.54O2 by rough coating with the superionic conductor Li7La3Zr2O12. ACS Appl Mater Interfaces 13:54906–54913
Tang Y, Han X, Zhang W, He Y (2020) La doping and coating enabled by one-step method for high performance Li1.2Mn0.54Ni0.13Co0.13O2 Li-rich cathode. Ionics 26:3737–3747
Zheng J, Deng S, Shi Z, Xu H, Xu H, Deng Y, Zhang Z, Chen G (2013) The effects of persulfate treatment on the electrochemical properties of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 cathode material. J Power Sources 221:108–113
Wu F, Zhang X, Zhao T, Li L, Xie M, Chen R (2015) Multifunctional AlPO4 coating for improving electrochemical properties of low-cost Li[Li0.2Fe0.1Ni0.15Mn0.55]O2 cathode materials for lithium-ion batteries. ACS Appl Mater Interfaces 7:3773–3781
Yano A, Shikano M, Ueda A, Sakaebe H, Ogumi Z (2017) LiCoO2 degradation behavior in the high-voltage phase transition region and improved reversibility with surface coating. J Electrochem Soc 164:A6116–A6122
Tao S, Kong F, Wu C, Su X, Xiang T, Chen S, Hou H, Zhang L, Fang Y, Wang Z, Chu W, Qian B, Song L (2017) Nanoscale TiO2 membrane coating spinel LiNi0.5Mn1.5O4 cathode material for advanced lithium-ion batteries. J Alloys Compd 705:413–419
Ghanty C, Dahiya PP, Basu RN, Chang J-K, Majumder SB (2015) Improvement of the electrochemical characteristics of lithium and manganese rich layered cathode materials: effect of surface coating. J Electrochem Soc 162:A1957–A1965
Guan P, Zhou L, Yu Z, Sun Y, Liu Y, Wu F, Jiang Y, Chu D (2020) Recent progress of surface coating on cathode materials for high-performance lithium-ion batteries. J Energy Chem 43:220–235
Song HG, Park K-S, Park YJ (2012) The effects of LaPO4 coating on the electrochemical properties of Li[Ni0.5Co0.2Mn0.3]O2 cathode material. Solid State Ion 225:532–537
Liu Y, Wang Q, Zhang Z, Dou A, Pan J, Su M (2016) Investigation the electrochemical performance of layered cathode material Li1.2Ni0.2Mn0.6O2 coated with Li4Ti5O12. Adv Powder Technol 27:1481–1487
Liu Y, Wang Q, Wang X, Wang T, Gao Y, Su M, Dou A (2015) Improved electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathode material with fast ionic conductor Li3VO4 coating. Ionics 21:2725–2733
Xu H, Deng S, Chen G (2014) Improved electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 by Mg doping for lithium ion battery cathode material. J Mater Chem A 2:15015–15021
Billaud J, Sheptyakov D, Sallard S, Leanza D, Talianker M, Grinblat J, Sclar H, Aurbach D, Novak P, Villevieille C (2019) Li/Fe substitution in Li-rich Ni, Co, Mn oxides for enhanced electrochemical performance as cathode materials. J Mater Chem A 7:15215–15224
Zhao Y, Liu J, Wang S, Ji R, Xia Q, Ding Z, Wei W, Liu Y, Wang P, Ivey DG (2016) Surface structural transition induced by gradient polyanion-doping in Li-rich layered oxides: implications for enhanced electrochemical performance. Adv Funct Mater 26:4760–4767
Wang Y, Yang Z, Qian Y, Gu L, Zhou H (2015) New insights into improving rate performance of lithium-rich cathode material. Adv Mater 27:3915–3920
Liu Y, Fan X, Zhang Z, Wu H-H, Liu D, Dou A, Su M, Zhang Q, Chu D (2019) Enhanced electrochemical performance of Li-rich layered cathode materials by combined Cr doping and LiAlO2 coating. ACS Sustain Chem Eng 7:2225–2235
Liu C, Neale ZG, Cao G (2016) Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater Today 19:109–123
Johnson CS, Kim JS, Lefief C, Li N, Vaughey JT, Thackeray MM (2004) The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3·(1-x)LiMn0.5Ni0.5O2 electrodes. Electrochem Commun 6:1085–1091
Myung ST, Izumi K, Komaba S, Sun YK, Yashiro H, Kumagai N (2005) Role of alumina coating on Li-Ni-Co-Mn-O particles as positive electrode material for lithium-ion batteries. Chem Mater 17:3695–3704
Dong S, Zhou Y, Hai C, Zeng J, Sun Y, Shen Y, Li X, Ren X, Qi G, Zhang X, Ma L (2019) Ultrathin CeO2 coating for improved cycling and rate performance of Ni-rich layered LiNi0.7Co0.2Mn0.1O2 cathode materials. Ceram Int 45:144–152
Ohzuku T, Ueda A, Nagayama M (1993) Electrochemistry and structural chemistry of LiNiO2 (R3m) for 4 volt secondary lithium cells. J Electrochem Soc 140:1862–1870
Liu Z, Wu Y, Huo H, Jian J, Sun D, Zhang X, Du C, Zuo P, Yin G, Ma Y (2022) Surface-phase engineering via lanthanum doping enables enhanced electrochemical performance of Li-rich layered cathode. ACS Appl Energy Mater 5:9648–9656
Assat G, Foix D, Delacourt C, Iadecola A, Dedryvere R, Tarascon J-M (2017) Fundamental interplay between anionic/cationic redox governing the kinetics and thermodynamics of lithium-rich cathodes. Nat Commun 8:2219
Xie Q, Zhao C, Hu Z, Huang Q, Chen C, Liu K (2015) LaPO4-coated Li1.2Mn0.56Ni0.16Co0.08O2 as a cathode material with enhanced coulombic efficiency and rate capability for lithium ion batteries. RSC Adv 5:77324–77331
Shi S, Wang T, Cao M, Wang J, Zhao M, Yang G (2016) Rapid self-assembly spherical Li1.2Mn0.56Ni0.16Co0.08O2 with improved performances by microwave hydrothermal method as cathode for lithium-ion batteries. ACS Appl Mater Interfaces 8:11476–11487
Penki TR, Shanmughasundaram D, Kishore B, Jeyaseelan AV, Subramani AK, Munichandraiah N (2016) Composite of Li-rich Mn, Ni and Fe oxides as positive electrode materials for Li-ion battery. J Electrochem Soc 163:A1493–A1502
Wang M, Chen L, Liu M, Chen Y, Gu Y (2020) Enhanced electrochemical performance of La-doped Li-rich layered cathode material. J Alloys Compd 848:156620
Yang L, Liang T, Zeng W, Zhu X, Chen Z, He H, Chen X, Yan W (2022) Improving the electrochemical performance of Li1.2Mn0.54Ni0.13Co0.13O2 cathode through sodium doping. Electrochim Acta 404:139775
Funding
This work is supported by the National Science Foundation of China (Nos. 51472119 and 21474053).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, L., Zheng, Z., Xia, W. et al. Improved rate performance of Li1.2Mn0.54Co0.13Ni0.13O2 Li-rich cathode by LaPO4 coating and Lanthanum doping. Ionics 29, 1311–1322 (2023). https://doi.org/10.1007/s11581-023-04918-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04918-y