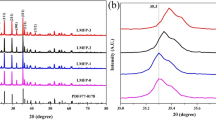
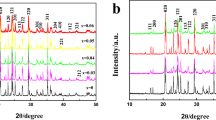
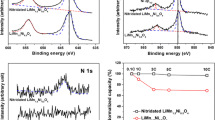
Abstract
Olivine LiMPO4 (M = Mn, Ni) cathode materials are being widely explored as potential cathode materials for lithium-ion batteries due to its good structural properties, high potential, and specific capacity reaching ~ 170 mAh g−1. Nevertheless, these cathode materials suffer poor electronic and ionic conductivity, stumbling its application in electrochemical devices. Thus, phospho-olivine cathodes research and development are continuously being studied directly proportional to its advanced applications. Among the various modifications to overcome the deficiency, this mini review explains the doping technique in LiMPO4 (M = Mn, Ni) cathode materials. Consequently, enhanced performances are discussed based on physical and electrochemical characteristics as well as a scalable application route.

















Similar content being viewed by others
Data availability
Not applicable.
References
Chen Y, Kang Y, Zhao Y et al (2021) A review of lithium-ion battery safety concerns: the issues, strategies, and testing standards. J Energy Chem 59:83–99. https://doi.org/10.1016/j.jechem.2020.10.017
Scrosati B, Hassoun J, Sun Y-K (2011) Lithium-ion batteries. A look into the future. Energy Environ Sci 4:3287–3295. https://doi.org/10.1039/C1EE01388B
Jyoti J, Singh BP, Tripathi SK (2021) Recent advancements in development of different cathode materials for rechargeable lithium ion batteries. J Energy Storage 43:103112. https://doi.org/10.1016/j.est.2021.103112
Lu K, Zhao C, Jiang Y (2021) Research progress of cathode materials for lithium-ion batteries. E3S Web Conf 233:01020. https://doi.org/10.1051/e3sconf/202123301020
Zaghib K, Guerfi A, Hovington P et al (2013) Review and analysis of nanostructured olivine-based lithium recheargeable batteries: status and trends. J Power Sources 232:357–369. https://doi.org/10.1016/j.jpowsour.2012.12.095
Aravindan V, Gnanaraj J, Lee YS, Madhavi S (2013) LiMnPO4 – a next generation cathode material for lithium-ion batteries. J Mater Chem A 1:3518–3539. https://doi.org/10.1039/c2ta01393b
Wani TA, Suresh G (2021) A comprehensive review of LiMnPO4 based cathode materials for lithium-ion batteries: current strategies to improve its performance. J Energy Storage 44:103307. https://doi.org/10.1016/j.est.2021.103307
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188. https://doi.org/10.1149/1.1837571
Korona KP, Papierska J, Kamińska M et al (2011) Raman measurements of temperature dependencies of phonons in LiMnPO4. Mater Chem Phys 127:391–396. https://doi.org/10.1016/j.matchemphys.2011.02.027
Tolganbek N, Yerkinbekova Y, Kalybekkyzy S et al (2021) Current state of high voltage olivine structured LiMPO4 cathode materials for energy storage applications: a review. J Alloys Compd 882:160774. https://doi.org/10.1016/j.jallcom.2021.160774
Ragupathi V, Panigrahi P, Nagarajan GS (2019) Enhanced electrochemical performance of nanopyramid-like LiMnPO4/C cathode for lithium–ion batteries. Appl Surf Sci 495:143541. https://doi.org/10.1016/j.apsusc.2019.143541
Ling J, Karuppiah C, Krishnan SG et al (2021) Phosphate polyanion materials as high-voltage lithium-ion battery cathode: a review. Energy Fuels 35:10428–10450. https://doi.org/10.1021/acs.energyfuels.1c01102
Zhang J, Luo SH, Sui LL et al (2021) Preparation of neodymium-doped LiMnPO4/C cathode by sol-gel method with excellent electrochemical performance for lithium-ion batteries. Int J Energy Res 45:10590–10598. https://doi.org/10.1002/er.6546
Zhu C, Wu Z, Xie J et al (2018) Solvothermal-assisted morphology evolution of nanostructured LiMnPO4 as high-performance lithium-ion batteries cathode. J Mater Sci Technol 34:1544–1549. https://doi.org/10.1016/j.jmst.2018.04.017
Zhang Y, Pan Y, Liu J et al (2015) Synthesis and electrochemical studies of carbon-modified LiNiPO4 as the cathode material of Li-ion batteries. Chem Res Chinese Univ 31:117–122. https://doi.org/10.1007/s40242-015-4261-9
Pavithra S, Priya A, Jayachandran M et al (2021) Influence of aloe-vera gel mediated CuO coated LiNiPO4 cathode material in rechargeable battery applications. Inorg Chem Commun 125:108459. https://doi.org/10.1016/j.inoche.2021.108459
Chang L, Bi X, Luo S et al (2021) Insight into structural and electrochemical properties of Mg-doped LiMnPO4/C cathode materials with first-principles calculation and experimental verification. Int J Energy Res 45:20715–20728. https://doi.org/10.1002/er.7135
Di Lecce D, Hu T, Hassoun J (2017) Electrochemical features of LiMnPO4 olivine prepared by sol-gel pathway. J Alloys Compd 693:730–737. https://doi.org/10.1016/j.jallcom.2016.09.193
Ong SP, Chevrier VL, Ceder G (2011) Comparison of small polaron migration and phase separation in olivine LiMnPO4 and LiFePO4 using hybrid density functional theory. Phys Rev B - Condens Matter Mater Phys 83:1–7. https://doi.org/10.1103/PhysRevB.83.075112
Rommel SM, Schall N, Brünig C, Weihrich R (2014) Challenges in the synthesis of high voltage electrode materials for lithium-ion batteries: a review on LiNiPO4. Monatshefte fur Chemie 145:385–404. https://doi.org/10.1007/s00706-013-1134-0
Rommel SM, Schall N, Brünig C, Weihrich R (2014) Challenges in the synthesis of high voltage electrode materials for lithium-ion batteries: a review on LiNiPO4. Monatsh Chem 145:385–404. https://doi.org/10.1007/s00706-013-1134-0
Michalska M, Lipińska L, Sikora A et al (2015) Structural and morphological studies of manganese-based cathode materials for lithium ion batteries. J Alloys Compd 632:256–262. https://doi.org/10.1016/j.jallcom.2014.12.266
El Khalfaouy R, Addaou A, Laajeb A, Lahsini A (2019) Solution combustion synthesis of LiMnPO4/C cathode material: the effect of four fuel sources on the electrochemical performances. Int J Hydrogen Energy 44:18272–18282. https://doi.org/10.1016/j.ijhydene.2019.05.129
Kumar PR, Venkateswarlu M, Misra M et al (2013) Enhanced conductivity and electrical relaxation studies of carbon-coated LiMnPO4 nanorods. Ionics (Kiel) 19:461–469. https://doi.org/10.1007/s11581-012-0778-9
Long Y, Zhang Z, Wu Z et al (2017) Microwave-assisted polyol synthesis of LiMnPO4/C and its use as a cathode material in lithium-ion batteries. Particuology 33:42–49. https://doi.org/10.1016/j.partic.2016.10.006
Liu J, Liu X, Huang T, Yu A (2013) Synthesis of nano-sized LiMnPO4 and in situ carbon coating using a solvothermal method. J Power Sources 229:203–209. https://doi.org/10.1016/j.jpowsour.2012.11.093
Li L, Lu J, Chen L, Xu H, Yang J, Qian Y (2013) Effect of different carbon sources on the electrochemical properties of rod-like LiMnPO4–C nanocomposites. RSC Adv 4:6847–6852. https://doi.org/10.1039/c3ra22862b
Li J, Luo S, Wang Q et al (2019) Facile fabrication of hierarchical LiMnPO4 microspheres for high-performance lithium-ion batteries cathode. J Electrochem Soc 166:A118–A124. https://doi.org/10.1149/2.0401902jes
Rajammal K, Sivakumar D, Duraisamy N et al (2016) Enhanced electrochemical properties of ZnO-coated LiMnPO4 cathode materials for lithium ion batteries. Ionics 22:1551–1556. https://doi.org/10.1007/s11581-016-1685-2
Fang H, Yi H, Hu C et al (2012) Effect of Zn doping on the performance of LiMnPO4 cathode for lithium ion batteries. Electrochim Acta 71:266–269. https://doi.org/10.1016/j.electacta.2012.03.160
Li X, Liu S, Jin H et al (2014) Ionothermal synthesis and electrochemical analysis of Fe doped LiMnPO4/C composites as cathode materials for lithium-ion batteries. J Alloys Compd 614:7–12. https://doi.org/10.1016/j.jallcom.2014.06.085
Sharmila V, Parthibavarman M (2021) Lithium manganese phosphate associated with MWCNT: enhanced positive electrode for lithium hybrid batteries. J Alloys Compd 858:157715. https://doi.org/10.1016/j.jallcom.2020.157715
Moon S, Muralidharan P, Kim DK (2012) Carbon coating by high-energy milling and electrochemical properties of LiMnPO4 obtained in polyol process. Ceram Int 38:S471–S475. https://doi.org/10.1016/j.ceramint.2011.05.042
Rajammal K, Sivakumar D, Duraisamy N et al (2017) Influences of sintering temperatures and crystallite sizes on electrochemical properties of LiNiPO4 as cathode materials via sol–gel route for lithium ion batteries. J Sol-Gel Sci Technol 83:12–18. https://doi.org/10.1007/s10971-017-4372-5
Fu X, Chang K, Li B et al (2017) Low-temperature synthesis of LiMnPO4/RGO cathode material with excellent voltage platform and cycle performance. Electrochim Acta 225:272–282. https://doi.org/10.1016/j.electacta.2016.12.161
Rajammal K, Sivakumar D, Duraisamy N et al (2016) Effect of sintering temperature on structural properties of LiMnPO4 cathode materials obtained by sol – gel method. J Sol-Gel Sci Technol 80:514–522. https://doi.org/10.1007/s10971-016-4111-3
Zhang J, Luo S, Wang Q et al (2017) Optimized hydrothermal synthesis and electrochemical performance of LiMnPO4/C cathode materials using high specific area spherical structure Li3PO4. J Alloys Compd 701:433–438. https://doi.org/10.1016/j.jallcom.2017.01.140
James Abraham J, Arro CRA, Tariq HA et al (2021) Sodium and lithium incorporated cathode materials for energy storage applications - a focused review. J Power Sources 506:230098. https://doi.org/10.1016/j.jpowsour.2021.230098
Tosun SG, Uzun D, Ye S (2021) Novel K+-doped Na0.6Mn0.35Fe0.35Co0.3O2 cathode materials for sodium-ion batteries : synthesis , structures , and electrochemical properties. J Solid State Electrochem 25:1271–1281. https://doi.org/10.1007/s10008-021-04906-0
Palaniyandy N (2020) Recent developments on layered 3d-transtition metal oxide cathode materials for sodium-ion batteries. Curr Opin Electrochem 21:319–326. https://doi.org/10.1016/j.coelec.2020.03.023
Lyu Y, Liu Y, Yu ZE et al (2019) Recent advances in high energy-density cathode materials for sodium-ion batteries. Sustain Mater Technol 21:e00098. https://doi.org/10.1016/j.susmat.2019.e00098
Zhu L, Ding G, Xie L et al (2019) Conjugated carbonyl compounds as high-performance cathode materials for rechargeable batteries. Chem Mater 31:8582–8612. https://doi.org/10.1021/acs.chemmater.9b03109
Zhu L, Pan C, Han Q et al (2022) The improved cycling stability and rate capability of Nb-doped NaV3O8 cathode for sodium-ion batteries. J Alloys Compd 890:161885. https://doi.org/10.1016/j.jallcom.2021.161885
Zhang J, Luo SH, Ren QX et al (2020) Tailoring the sodium doped LiMnPO4/C orthophosphate to nanoscale as a high-performance cathode for lithium ion battery. Appl Surf Sci 530:146628. https://doi.org/10.1016/j.apsusc.2020.146628
El Khalfaouy R, Addaou A, Laajeb A, Lahsini A (2019) Synthesis and characterization of Na-substituted LiMnPO4 as a cathode material for improved lithium ion batteries. J Alloys Compd 775:836–844. https://doi.org/10.1016/j.jallcom.2018.10.161
Rajammal K, Sivakumar D, Duraisamy N et al (2017) Na-doped LiMnPO4 as an electrode material for enhanced lithium ion batteries. Bull Mater Sci 40:171–175. https://doi.org/10.1007/s12034-017-1365-5
Prabu M, Selvasekarapandian S, Kulkarni AR et al (2011) Structural, dielectric, and conductivity studies of yttrium-doped LiNiPO4 cathode materials. Ionics 17:201–207. https://doi.org/10.1007/s11581-011-0535-5
Yang H, Fu C, Sun Y et al (2020) Fe-doped LiMnPO4@C nanofibers with high Li-ion diffusion coefficient. Carbon N Y 158:102–109. https://doi.org/10.1016/j.carbon.2019.11.067
Hong Y, Tang Z, Hong Z, Zhang Z (2014) LiMn1-xFexPO4 (x = 0, 0.1, 0.2) nanorods synthesized by a facile solvothermal approach as high performance cathode materials for lithium-ion batteries. J Power Sources 248:655–659. https://doi.org/10.1016/j.jpowsour.2013.09.123
Yang W, Bi Y, Qin Y et al (2015) LiMn0.8Fe0.2PO4/C cathode material synthesized via co-precipitation method with superior high-rate and low-temperature performances for lithium-ion batteries. J Power Sources 275:785–791. https://doi.org/10.1016/j.jpowsour.2014.11.063
Liao L, Wang H, Guo H et al (2015) Facile solvothermal synthesis of ultrathin LiFexMn1-xPO4 nanoplates as advanced cathodes with long cycle life and superior rate capability. J Mater Chem A Mater Energ Sustain 3:19368–19375. https://doi.org/10.1039/C5TA05358G
Xu S, Lv X-Y, Wu Z et al (2017) Synthesis of porous-hollow LiMn0.85Fe0.15PO4/C microspheres as a cathode material for lithium-ion batteries. Powder Technol 308:94–100. https://doi.org/10.1016/j.powtec.2016.12.002
Zou B, Yu R, Deng M et al (2016) Solvothermal synthesized LiMn1-xFexPO4@C nanopowders with excellent high rate and low temperature performances for lithium-ion batteries. RSC Adv 6:52271–52278. https://doi.org/10.1039/C6RA12472K
Ruan T, Wang Bo, Wang F, Song R, Fan Jin Yu, Zhou DW, SD, (2019) Stabilizing the structure of LiMn0.5Fe0.5PO4 via formation of concentration-gradient hollow spheres with Fe-rich surfaces. Nanoscale 11:3933–3944. https://doi.org/10.1039/b000000x
Hu L, Qiu B, Xia Y et al (2014) Solvothermal synthesis of Fe-doping LiMnPO4 nanomaterials for Li-ion batteries. J Power Sources 248:246–252. https://doi.org/10.1016/j.jpowsour.2013.09.048
Lei Z, Naveed A, Lei J et al (2017) High performance nano-sized LiMn1-xFexPO4 cathode materials for advanced lithium-ion batteries. RSC Adv 7:43708–43715. https://doi.org/10.1039/C7RA08993G
Oukahou S, Elomrani A, Maymoun M et al (2022) Investigation of LiMn1-xMxPO4 (M = Ni, Fe) as cathode materials for Li-ion batteries using density functional theory. Comput Mater Sci 202:111006. https://doi.org/10.1016/j.commatsci.2021.111006
Gan Y, Chen C, Liu J et al (2015) Enhancing the performance of LiMnPO4/C composites through Cr doping. J Alloys Compd 620:350–357. https://doi.org/10.1016/j.jallcom.2014.09.160
Bibi S, Khan A, Khan S et al (2022) Synthesis of Cr doped LiMnPO4 cathode materials and investigation of their dielectric properties. Int J Energy Res 46:810–821. https://doi.org/10.1002/er.7205
Dai E, Fang H, Yang B et al (2015) Synthesis of vanadium doped LiMnPO4 by an improved solid-state method. Ceram Int 41:8171–8176. https://doi.org/10.1016/j.ceramint.2015.03.035
Vasquez FA, Calderón JA (2019) Vanadium doping of LiMnPO4 cathode material : correlation between changes in the material lattice and the enhancement of the electrochemical performance. Electrochim Acta 325:1–10. https://doi.org/10.1016/j.electacta.2019.134930
Huang Q, Wu Z, Su J et al (2016) Synthesis and electrochemical performance of Ti – Fe co-doped LiMnPO4/C as cathode material for lithium-ion batteries. Ceram Int 42:11348–11354. https://doi.org/10.1016/j.ceramint.2016.04.057
Khalfaouy ELR, Turan S, Dermenci KB et al (2019) Nickel-substituted LiMnPO4/C olivine cathode material: combustion synthesis, characterization and electrochemical performances. Ceram Int 45:17688–17695. https://doi.org/10.1016/j.ceramint.2019.05.336
El Khalfaouy R, Turan S, Rodriguez MA et al (2020) Solution combustion synthesis and electrochemical properties of yttrium-doped LiMnPO4/C cathode materials for lithium ion batteries. J Rare Earths 38:976–982. https://doi.org/10.1016/j.jre.2019.06.004
Zhang J, Luo S, Wang Q et al (2017) Yttrium substituting in Mn site to improve electrochemical kinetics activity of sol-gel synthesized LiMnPO4/C as cathode for lithium ion battery. J Solid State Electrochem 21:3189–3194. https://doi.org/10.1007/s10008-017-3662-8
Wang R, Zheng J, Feng X et al (2020) Highly [010]-oriented, gradient Co-doped LiMnPO4 with enhanced cycling stability as cathode for Li-ion batteries. J Solid State Electrochem 24:511–519. https://doi.org/10.1007/s10008-019-04485-1
El-Khalfaouy R, Turan S, Rodriguez MA et al (2021) A scalable approach for synthesizing olivine structured LiMn1−xCoxPO4/C high-voltage cathodes. J Appl Electrochem 51:681–689. https://doi.org/10.1007/s10800-020-01528-8
Liu J, Wang J, Chen Q, Zhong S (2021) Mg-doped LiMnPO4/C cathode materials for enhanced lithium storage performance. Mater Technol 36:153–158. https://doi.org/10.1080/10667857.2020.1735076
Wang L, Zhang H, Liu Q et al (2018) Modifying high-voltage olivine-type LiMnPO4 cathode via Mg-substitution in high-orientation crystal. ACS Appl Energy Mater 1:5928–5935. https://doi.org/10.1021/acsaem.8b00923
Nageswara Rao B, Narsimulu D, Satyanarayana N (2022) Effect of Mg doping on the electrical, dielectric and relaxation properties of LiMnPO4 nanoparticles. Indian J Phys 96:1017–1023. https://doi.org/10.1007/s12648-021-02023-2
Luo S, hua, Sun Y, Bao S, et al (2019) Synthesis of Er-doped LiMnPO4/C by a sol-assisted hydrothermal process with superior rate capability. J Electroanal Chem 832:196–203. https://doi.org/10.1016/j.jelechem.2018.10.062
Rajammal K, Sivakumar D, Duraisamy N et al (2016) Structural and electrochemical characterizations of LiMn1-xAl0.5xCu0.5xPO4 (x=0.0, 0.1, 0.2) cathode materials for lithium ion batteries. Mater Lett 173:131–135. https://doi.org/10.1016/j.matlet.2016.03.046
Chen L, Yuan Y, Feng X, Li M (2012) Enhanced electrochemical properties of LiFe1-xMnxPO4/C composites synthesized from FePO4.2H2O nanocrystallites. J Power Sources 214:344–350. https://doi.org/10.1016/j.jpowsour.2012.04.089
Duan J, Hu G, Cao Y et al (2016) Synthesis of high-performance Fe-Mg-co-doped LiMnPO4/C via a mechano-chemical liquid-phase activation technique. Ionics 22:609–619. https://doi.org/10.1007/s11581-015-1582-0
Bi X, Chang L, Luo S et al (2022) Based on first-principles calculation, study on the synthesis, and performance of Fe–Ni co-doped LiMnPO4/C as cathode material for lithium-ion batteries. Ionics 28:577–591. https://doi.org/10.1007/s11581-021-04344-y
Xiang W, Zhong Y, Tang Y et al (2015) Improving the electrochemical kinetics of lithium manganese phosphate via co-substitution with iron and cobalt. J Alloys Compd 635:180–187. https://doi.org/10.1016/j.jallcom.2015.02.049
Yi H, Hu C, He X, Xu H (2015) Electrochemical performance of LiMnPO4 by Fe and Zn co-doping for lithium-ion batteries. Ionics (Kiel) 21:667–671. https://doi.org/10.1007/s11581-014-1238-5
Devi LS, Babu KV, Madhavilatha B et al (2018) Structural and electrochemical characterizations of nanostructured olivine LiNi1-xCoxPO4 (x=0 and 0.5) cathode materials for lithium-ion batteries. South African J Chem Eng 25:42–47. https://doi.org/10.1016/j.sajce.2017.12.003
Vijaya Babu K, Seeta Devi L, Veeraiah V, Anand K (2016) Structural and dielectric studies of LiNiPO4 and LiNi0.5Co0.5PO4 cathode materials for lithium-ion batteries. J Asian Ceram Soc 4:269–276. https://doi.org/10.1016/j.jascer.2016.05.001
Karthickprabhu S, Vikraman D, Kathalingam A et al (2019) Electrochemical and cycling performance of neodymium (Nd3+) doped LiNiPO4 cathode materials for high voltage lithium-ion batteries. Mater Lett 237:224–227. https://doi.org/10.1016/j.matlet.2018.11.102
Tao Y, Zhu B (2021) Yttrium ion doping effect on electrochemical performance of LiNiPO4 materials. Ionics 27:2909–2914. https://doi.org/10.1007/s11581-021-04089-8
Zhang J, Luo SH, Sui LL et al (2018) Co-precipitation assisted hydrothermal method to synthesize Li0.9Na0.1Mn0.9Ni0.1PO4/C nanocomposite as cathode for lithium ion battery. J Alloys Compd 768:991–994. https://doi.org/10.1016/j.jallcom.2018.07.309
Zhang J, Luo S, Ren Q et al (2021) Preparation and electrochemical performance of Na+ and Co2+ co-doped Li0.9Na0.1Mn1-xCoxPO4/C cathode material for Li-ion battery. Ionics 27:3251–3257. https://doi.org/10.1007/s11581-021-04102-0
Han CC, Yao X, Tian H et al (2021) Synthesis and electrochemical behavior of Na+ and Zr4+ doped LiMnPO4/C as potential cathode material for Li-ion batteries. Int J Electrochem Sci 16:1–10. https://doi.org/10.20964/2021.07.69
Hu CL, Yi HH, Wang FX et al (2014) Boron doping at P-site to improve electrochemical performance of LiMnPO4 as cathode for lithium ion battery. J Power Sources 255:355–359. https://doi.org/10.1016/j.jpowsour.2013.12.040
Clemens O, Bauer M, Haberkorn R et al (2012) Synthesis and characterization of vanadium-doped LiMnPO4- compounds: LiMn(PO4)x(VO4)1–x (0.8 ≤ x ≤ 1.0). Chem Mater 24:4717–4724. https://doi.org/10.1021/cm303005d
Author information
Authors and Affiliations
Contributions
K. Rajammal wrote the original draft. All authors participated in writing, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajammal, K., Ramesh, K., Ramesh, S. et al. Doped olivine LiMPO4 (M = Mn/Ni) derivatives as potential cathode materials for Lithium-ion batteries: a mini review. Ionics 29, 895–916 (2023). https://doi.org/10.1007/s11581-023-04894-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-04894-3