Abstract
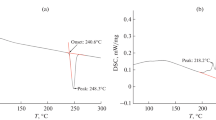
The properties of high-temperature phases of Et4NHSO4 and the temperature ranges of their stability were investigated. The sequence of the reversible phase transitions at 147°C and 160°C with the enthalpies of − 3.28 J/g and − 8.99 J/g, respectively, was observed. It was shown that at 160° C the I41/acd phase with the unit cell parameters a = 14.0430(8) Å, c = 25.686(3) Å with higher degree of sulfate tetrahedra disordering was formed. This phase exists up to the melting of Et4NHSO4, 245°C. The proton conductivity of Et4NHSO4 was determined firstly and the temperature dependence varies widely from 10−8 S/cm at 90°C up to ~ 10−2 S/cm at the melting. The conductivity of the Et4NHSO4 is rather low and does not exceed 5*10−6 S/cm at temperatures below 210°C. The structural closed dimers of hydrogen-bonded sulfate anions at low temperatures hinder the effective proton transfer. Spectral characteristics of room-temperature phase also have been discussed.






Similar content being viewed by others
References
Bureš F (2019) Quaternary ammonium compounds: simple in structure, complex in application. Top Curr Chem 377:14
MacFarlane DR, Forsyth M (2001) Plastic crystal electrolyte materials: new perspectives on solid state ionics. Adv Mater 13:957–966
Pring JM, Howlett PC, MacFarlane DR, Forsyth M (2010) Organic ionic plastic crystals: recent advances. J Mater Chem 20:2056–2062
Baranowski B, Lipkowski J, Lunden A (1995) On the phase transitions of cesium hydrogen sulfate (CsHSO4): J Solid State Chem 117:412–413
Colomban Ph, Pham-Thi M, Novak A (1987) Influence of thermal and mechanical treatment and of water on structural phase-transitions in CsHSO4. Solid State Ionics 24:193–198
Chisholm CRI, Haile SM (2000) X-ray structure refinement of CsHSO4 in phase II: Materials Research Bulletin. Mat Res Bull 35:999–1005
Kemnitz E, Troyanov SI (1998) Hydrogen bonding systems in acid metal sulfates and selenates. In: Hargittai M, Hargittai I (eds) Advances in molecular structure research, 1st edn. JAI Press, Stamford CT, pp 79–113
Itoh K, Ozaki T, Nakamura E (1981) Structure of cesium hydrogen sulfate. Acta Crystallogr B 37:1908–1910
Colomban Ph (1992) Proton conductors: solids, membranes and gels – materials and devices. ed. Ph Colomban. Cambridge: Cambridge Univ Press p 581
Baranov AI (2003) Crystals with disordered hydrogen bond networks and superionic conductivity. Rev Crystallogr 48:1081–1107
Zakharov MA, Troyanov SI, Rybakov VB, Aslanov LA, Kemnitz E (2001) Crystal structures of [N(CH3)4](HSeO4)] at 298, 363, and 380 K. Crystallogr Rep 46:974–979
Baran J, Sledz M, Drozd M, Pietraszko A, Haznar A, Ratajczak H (2000) Structural, vibrational and DSC investigations of the tetraethylammonium hydrogen selenate crystals. J Mol Struct 526:361–437
Stejskal J, Havlíček D, Císařová I, Matulková I (2017) Vibrational spectroscopic and X-ray single crystal diffraction investigation of tetra-n-alkylammonium hydrogen selenates. J Chem Crystallogr 47:59–68
Rivera J-P, Speziali NL, Berger H, Arend H, Schmid H (1990) Optical, X-ray and DSC measurements on N(CH3)4HSO4. Ferroelectrics 105:183–188
Kossev K, Sbirkova H, Petrova N, Shivachev B, Nikolova R (2013) Crystal structure and properties of urea and thiourea adducts of tetraalkyl ammonium hydrogen sulphate. Bul Chem Commun 45:446–454
Light ME, Gale PA, Hursthouse MB (2001) Anion-anion dimerization in tetrabutylammonium hydrogen sulfate. Acta Cryst E57:o705–o706
Speziali NL, Chapuis G (1991) Phase transitions in N(CH3)4HSO4: a novel compound with an lncommensurate phase. Acta Cryst B47:757–766
Blinc R, Lahajnar G, Zupancic I, Arend H (1984) Anomalous acid proton self-diffusion in N(CH3)4HSO4 - a candidate for proton superionic conductivity. Solid State Commun 51:751–752
Ponomareva VG, Bagryantseva IN, Uvarov NF (2021) Electrotransport and thermal properties of tetrabutylammonium hydrogen sulfate. Ionics 27:2067–2071
Coelho AA, Evans JSO, Evans IR, Kern A, Parsons S (2011) The TOPAS symbolic computation system. Powder Diffr 26:S22
Baranowski B, Friesel M, Lundén A (1986) Preparation of different solid CsHSO4 phases by means of sample treatment. Z Naturforsch 41A:733–736
Jirak Z, Dlouha M, Vratislav S, Balagurov AM, Beskrovnyi AI, Gordelii VI, Datt ID, Shuvalov LA (1987) A neutron-diffraction study of the superioriic phase in CsHSO4. Physica Status Solidi A-Applied Res 100:K117–K122
Chisholm CRI, Haile SM (2000) X-ray structure refinement of CsHSO4 in phase II. Mater Res Bull 35:999–1005
Colomban Ph (1988) Proton transfer and superionic conductivity in solids and gels J Molec Struct 81:1–47
Varma V, Rangavital N, Rao CNR (1993) A study of superionic CsHSO4 and Cs1-xLixHSO4 by vibrational spectroscopy and X-ray diffraction. J Solid State Chem 106:164–173
Burgina EB, Ponomareva VG, Baltahinov VP, Kostrovskiy VG (2005) Spectroscopic study of the structure and mechanism of proton conductivity of CsHSO4 and CsHSO4/SiO2 composites. Russ J Struct Chem 46:630–640
Ribeiro MCC (2020) Strong anion–anion hydrogen bond in the ionic liquid 1-ethyl-3-methylimidazolium hydrogen sulfate. J Mol Liq 310:113178
Paschoal VH, Faria LFO, Ribeiro MCC (2017) Vibrational spectroscopy of ionic liquids. Chem Rev 117:7053–7112
Novak A (1974) Hydrogen bonding in solids. Correlation of spectroscopic and crystallographic data, Large Molecules. Structure and Bonding, 18, Springer, Berlin, Heidelberg 177–216
Acknowledgements
The authors thank PhD Gerasimov K. B. for the investigation of the thermal properties of Et4NHSO4.
Funding
This work was supported by the Russian Science Foundation grant no. 20–13-00302.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ponomareva, V.G., Bagryantseva, I.N., Bulina, N.V. et al. Proton conductivity and structural properties of tetraethylammonium hydrogen sulfate. Ionics 28, 4667–4674 (2022). https://doi.org/10.1007/s11581-022-04710-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-022-04710-4