Abstract
The precursor of Co metal organic framework (MOF) was first synthesized by hydrothermal method; then, the optimal Co-MOF was pyrolyzed in a nitrogen atmosphere to obtain the derivative (Co/C). The as-prepared materials were finally applied to water splitting to investigate their oxygen evolution reaction (OER) performance. A series of electrochemical tests such as linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS) show that Co/C (700 °C) exhibits lowest overpotential (420 mV) and charge transfer resistance (Rct = 5.25 Ω), smallest Tafel slope (119 mV dec−1) as compared to other relevant materials. After the cyclic life test of 10,000 s, the current density of Co/C (700 °C) remained at 94.88%. In addition, the comparison of the OER performance between Co/C (700 °C) with other reported cobalt-based materials illustrates that Co/C (700 °C) has lower overpotential, and its Tafel slope is also comparable with these reported cobalt-based materials. Therefore, Co/C (700 °C) has broad application prospect in oxygen-evolution reaction.
Graphical abstract
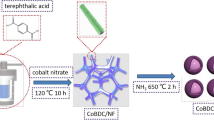
Scheme 1 Fabrication Co/C derived from Co-MOF and brief schematic of OER.










Similar content being viewed by others
References
Ding D, Shen K, Chen X-D, Chen H-R, Chen J-Y, Fan T, Wu R-F, Li Y-W (2018) Multi-level architecture optimization of MOF-templated Co-based nanoparticles embedded in hollow N-doped carbon polyhedra for efficient OER and ORR. ACS Catal 8:7879–7888
Yan L, Dai P, Wang Y, Gu X, Li L, Cao L, Zhao X (2017) In situ synthesis strategy for hierarchically porous Ni2P polyhedrons from MOFs templates with enhanced electrochemical (properties for hydrogen evolution. ACS Appl Mater Interfaces 9:11642–11650
Gao J, Huang Q, Wu Y, Lan Y-Q, Chen B (2021) Metal–organic frameworks for photo/electrocatalysis. Adv Energy Sustain Res 2:2100033
Yang J, Zhang F, Wang X, He D, Wu G, Yang Q, Hong X, Wu Y, Li Y (2016) Porous molybdenum phosphide nano-octahedrons derived from confined phosphorization in UIO-66 for efficient hydrogen evolution. Angew Chem 55:12854–12858
Xia C, Jiang Q, Zhao C, Hedhili MN, Alshareef HN (2016) Selenide-based electrocatalysts and scaffolds for water oxidation applications. Adv Mater 28:77–85
Novoselov KS, Jiang D, Schedin F, Booth TJ, Khotkevich VV, Morozov SV, Geim AK (2005) Two-dimensional atomic crystals. Proc Natl Acad Sci U S A 102:10451–10453
Li Y, Zhao T, Lu M, Wu Y, Xie Y, Xu H, Zhang Q (2019) Enhancing oxygen evolution reaction through modulating electronic structure of trimetallic electrocatalysts derived from metal–organic frameworks. Small 15:1901940
Liu Q, Xie L, Shi X, Du G, Asiri AM, Luo Y, Sun X (2018) High-performance water oxidation electrocatalysis enabled by a Ni-MOF nanosheet array. Inorg Chem Front 5:1570–1574
Wang C, Liu X, Keser DN, Du G, Asiri AM, Luo Y, Sun X (2016) Applications of water stable metal-organic frameworks. Chem Soc Rev 45:5107–5108
Zhang H (2015) Ultrathin two-dimensional nanomaterials. ACS Nano 9:9451–9469
Barreto L, Makihira A, Riahi K (2003) The hydrogen economy in the 21st century: a sustainable development scenario. Int J Hydrogen Energy 28:267–284
Meilikhov M, Yusenko K, Esken D, Turner S, Tendeloo GV, Fischer RA (2010) Metals@MOFs-loading MOFs with metal nanoparticles for hybrid functions. Eur J Inorg Chem 24:3701–3714
Wang Y, Zhang Z, Liu X, Ding F, Zou P, Wang X, Zhao Q, Rao H (2018) MOF-derived NiO/NiCo2O4 and NiO/NiCo2O4-rGO as highly efficient and stable electrocatalysts for oxygen evolution reaction. ACS Sustain Chem Eng 6:12511–12521
Sun D, Ye L, Sun F, García H, Li Z (2017) From mixed-metal MOFs to carbon-coated core–shell metal alloy@metal oxide solid solutions: transformation of Co/Ni-MOF-74 to CoxNi1–x@ CoyNi1–yO@C for the oxygen evolution reaction. Inorg Chem 56:5203–5209
Duan J-J, Chen S, Zhao C (2017) Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat Commun 8:1–7
Flugel EA, Lau VW, Schlomberg H, Glaum R, Lotsch BV (2016) Homonuclear mixed-valent cobalt imidazolate framework for oxygen-evolution electrocatalysis. Chem A Eur J 22:3676
Wang C-H, Liu X-L, Demir NK, Chen J-P, Li K (2016) Applications of water stable metaleorganic frameworks. Chem Soc Rev 45:5107
Wu H-B, (David) Lou X-W (2017) Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: promises and challenges. Sci Adv 3:9252
Xie A-J, Zhang J, Tao X (2019) Nickel-based MOF derived Ni@NiO/NeC nanowires with core-shell structure for oxygen evolution reaction. Electrochim Acta 324:134814
Wu Y, Li Y, Gao J, Zhang Q (2021) Recent advances in vacancy engineering of metal-organic frameworks and their derivatives for electrocatalysis. SusMat 1:66–87
Zhong L, Bao Y, Feng L (2019) Fe-doping effect on CoTe catalyst with greatly boosted intrinsic activity for electrochemical oxygen evolution reaction. Electrochim Acta 321:134656
Zhang C, Tang B, Gu X, Feng L (2019) Surface chemical state evaluation of CoSe2 catalysts for the oxygen evolution reaction. Chem Commun 55:10928–10931
Li M, Ying G, Chang Y, Xiaocong G, Tian J, Xiang W, Feng L (2021) Iron doped cobalt fluoride derived from CoFe layered double hydroxide for efficient oxygen evolution reaction. Chem Eng J 425:130686. https://doi.org/10.1016/j.cej.2021.130686
Feng L, Ding R, Chen Y, Wang J, Xu L (2020) Zeolitic imidazolate framework-67 derived ultra-small CoP particles incorporated into n-doped carbon nanofiber as efficient bifunctional catalysts for oxygen reaction. J Power Sources 452:227837
Schejn A, Balan L, Falk V, Aranda L, Medjahdi G, Schneider R (2014) Controlling ZIF-8 nano-and microcrystal formation and reactivity through zinc salt variations. CrystEngComm 16:4493–4500
Forgie R, Bugosh G, Neyerlin KC, Liu Z, Strasser P (2010) Bimetallic Ru electrocatalysts for the OER and electrolytic water splitting in acidic media. Electrochem Solid State Lett 13:B36–B39
Cao F, Gan M, Ma L, Li X, Yan F, Ye M, Zhai Y, Zhou Y (2017) Hierarchical sheet-like Ni–Co layered double hydroxide derived from a MOF template for high-performance supercapacitors. Synth Met 234:154–160
Danilovic N, Subbaraman R, Chang KC, Chang S-H, Kang Y-J, Snyder J, Paulikas AP, Strmcnik D, Kim KT, Myers D, Stamenkovic VR, Markovic NM (2014) Using surface segregation to design stable Ru-Ir oxides for the oxygen evolution reaction in acidic environments. Angew Chem Int Ed 53:14016–14021
Song G, Wang Z, Wang L, Li G, Huang M, Yin F (2006) Preparation of MOF(Fe) and its catalytic activity for oxygen reduction reaction in an alkaline electrolyte. Chin J Catal 35:185–195
Kurfiřtová L, Seo YK, Hwang YK, Chang J-S, Cejka J (2012) High activity of iron containing metal-organic-framework in acylation of p-xylene with benzoyl chloride. Catal Today 179:85–90
Yan W-N, Yang Z-R, Bian W-Y, Yang Y-Y (2015) FeCo2O4/hollow graphene spheres hybrid with enhanced electrocatalytic activities for oxygen reduction and oxygen evolution reaction. Carbon 92:74–83
Mousavi DS, Asen P, Shahrokhian S, Irajizad A (2019) Three-dimensional hybrid of iron–titanium mixed oxide/nitrogen-doped graphene on Ni foam as a superior electrocatalyst for oxygen evolution reaction. J Colloid Interface Sci 563:241–251
Karthikeyan N, Prince JJ, Ramalingam S, Periandy S (2014) Electronic [UV–visible] and vibrational [FT-IR, FT-Raman] investigation and NMR–mass spectroscopic analysis of terephthalic acid using quantum Gaussian calculations. Spectrochim Acta A Mol Biomol Spectrosc 139:229–242
Abedini H, Shariati A, Khosravi-Nikou MR (2020) Adsorption of propane and propylene on M-MOF-74 (M=Cu, Co): Equilibrium and kinetic study. Chem Eng Res Des 153:96–106
Wang J, Ni Y (2020) Ammonium molybdate-assisted shape-controlled synthesis of fluorescent Co(II)-based MOFs nanoflakes as highly-sensitive probes for selective detection of vanillin in milk powders. Mater Res Bull 123:110721
Zha Q-Q, Yuan F-F, Qin G-X, Ni Y (2020) Cobalt-based MOF-on-MOF two-dimensional heterojunction nanostructures for enhanced oxygen evolution reaction electrocatalytic activity. Inorg Chem 59:1295–1305
Yang J, Zhang F, Wang X, He D, Wu G, Yang Q (2016) Porous molybdenum phosphide nano-octahedrons derived from confined phosphorization in UIO-66 for efficient hydrogen evolution. Angew Chem 55:12854–12858
He P, Yu X-Y, Lou X-W (2017) Carbon-incorporated nickel-cobalt mixed metal phosphide nanoboxes with enhanced electrocatalytic activity for oxygen evolution. Angew Chem Int Ed 56:3897–3900
Yu X, Feng Y, Guan B, Lou X-W, Pailk U (2016) Carbon coated nickel phosphides porous nanoplates for highly efficient oxygen evolution reaction. Energy Environ Sci 9:1246–1250
Wang M, Wang P, Li C, Li H, Jin Y (2018) Boosting electrocatalytic oxygen evolution performance of ultrathin Co/Ni-MOF nanosheets via plasmon-induced hot carriers. ACS Appl Mater Interfaces 10:37095–37102
Khan SB, Khan SA, Asiri AM (2016) A fascinating combination of Co, Ni and Al nanomaterial for oxygen evolution reaction. Appl Surf Sci 370:445–451
Yu C, Liu Z, Han X, Huang H, Zhao C, Yang J, Qiu J (2016) NiCo-layered double hydroxides vertically assembled on carbon fiber papers as binder-free high-active electrocatalysts for water oxidation. Carbon 110:1–7
Liu G, Liu J, Liu B, Yang C, Qian D, Li J (2018) Ketjen black carbon supported CoO@Co−N−C nanochains as an efficient electrocatalyst for oxygen evolution. Int J Hydrogen Energy 43:22942–22948
Yuan Y, Sun L, Li Y, Zhan W, Wang X, Han X (2020) Synergistic modulation of active sites and charge transport: N/S Co-doped C encapsulated NiCo2O4/NiO hollow microrods for boosting oxygen evolution catalysis. Inorg Chem 59:4080–4089
Zou Z, Wang T, Zhao X, Jiang W, Pan H, Gao D, Xu C (2019) Expediting in-situ electrochemical activation of two-dimensional metal–organic frameworks for enhanced OER intrinsic activity by iron incorporation. ACS Catal 9:7356–7364
Zhang W, Li D, Zhang L, She X, Yang D (2019) NiFe-based nanostructures on nickel foam as highly efficiently electrocatalysts for oxygen and hydrogen evolution reactions. J Energy Chem 39:39–53
Béjar J, Álvarez-Contreras L, Ledesma-García J, Arjona N, Arriaga LG (2019) Electrocatalytic evaluation of Co3O4 and NiCo2O4 rosettes-like hierarchical spinel as bifunctional materials for oxygen evolution (OER) and reduction (ORR) reactions in alkaline media. J Electroanal Chem 847:113190
Zhang C, Duan N, Jiang L, Xu F (2018) The impact mechanism of Mn2+ ions on oxygen evolution reaction in zinc sulfate electrolyte. J Electroanal Chem 811:53–61
Peng L, Shah SSA, Wei Z (2018) Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction. Chin J Catal 39:1575–1593
Li D, Xu H-Q, Jiao L, Jiang H-L (2019) Metal-organic frameworks for catalysis: state-of-the-art, challenges, and opportunities. J Energy Chem 1:100005
Jayaraman T, Murthy AP, Elakkiya V, Chandrasekaran S, Nithyadharseni P, Khan Z, Senthil AR, Shanker R, Raghavender M, Kuppusami P, Jagannathan M, Ashokkumar M (2018) Recent development on carbon based heterostructures for their applications in energy and environment: a review. J Ind Eng Chem 64:16–59
Asiri AM, Akhtar K, Khan SB (2019) Cobalt oxide nanocomposites and their electrocatalytic behavior for oxygen evolution reaction. Ceram Int 45:13340–13346
Kandiel TA (2019) Iron-incorporated NiS/Ni(OH)2 composite as an efficient electrocatalyst for hydrogen evolution reaction from water in a neutral medium. Appl Catal A Gen 586:117226
Forster JC, Marcu LG, Bezak E (2019) Approaches to combat hypoxia in cancer therapy and the potential for in silico models in their evaluation. Phys Med Eur J Med Phys 64:145–156
Funding
This work was supported by Changzhou Science and Technology Support Plan (Social Development, CE20205052) (Jiangsu Province, China).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiabing Hu and Haoye Wang contributed equally to this work and should be regarded as first joint authors.
Rights and permissions
About this article
Cite this article
Hu, X., Wang, H., Qi, S. et al. Co/C nanomaterial derived from Co metal–organic framework for oxygen evolution reaction. Ionics 28, 813–821 (2022). https://doi.org/10.1007/s11581-021-04376-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04376-4