Abstract
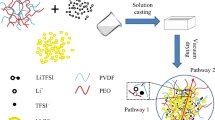
Commercial lithium-ion batteries have safety risks due to the use of electrolyte-containing flammable organic solvents. The development of all solid-state lithium ion batteries with solid electrolyte is a feasible technical way to improve the safety of batteries. In this work, PVDF-based composite solid-state electrolytes (CSEs) were prepared by coupling Li6.4La3Zr1.4Ta0.6O12 (LLZTO) and polyvinylidene fluoride (PVDF). The electrochemical performance and interfacial compatibility between composite electrolyte and electrode were improved by the combination of appropriate amount of Li-ion-conducting particles and the insulating polymer. The corresponding solid-state battery of LiFePO4 |PVDF-LLZTO-CSE| Li is fabricated and delivers initial discharge capacity of 162.4 mAh g-1, and coulomb efficiency is approximately 98.5%. And the solid-state battery exhibits satisfactory rate capability and cycling stability at room temperature. This study suggests that a combination of the lithium garnet particles and insulating polymer is one of the promising methods to solve the problem of liquid electrolyte.





Similar content being viewed by others
References
Armand M, Tarascon JM (2008) Building better batteries: researchers must find a sustainable way of providing the power our modern lifestyles demand[J]. Nature 451(7179):652–657
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries[J]. Nature 414(6861):359–367
Scrosati B, Hassoun J, Sun YK (2011) Lithium-ion batteries. A look into the future[J]. Energy Environ Sci 4(9):3287–3295
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries [J]. Chem Mater 22(3):587–603
Yue L, Ma J, Zhang J, Zhao J, Dong S, Liu Z, Cui G, Chen L (2016) All solid-state polymer electrolytes for high-performance lithium ion batteries[J]. Energy Storage Mater 5:139–164
Wiers BM, Foo ML, Balsara NP et al (2011) A solid lithium electrolyte via addition of lithium isopropoxide to a metal-organic framework with open metal sites. [J]. J Am Chem Soc 133(37):14522
Wang X, Lu X, Liu B, Chen D, Tong Y, Shen G (2014) Flexible energy-storage devices: design consideration and recent progress[J]. Adv Mater 26:4763–4782
Xu X, Wen Z, Yang X, Zhang J, Gu Z (2006) High lithium ion conductivity glass-ceramics in Li2O-Al2O3-TiO2-P2O5 from nanoscaled glassy powders by mechanical milling. Solid State Ionics 177:2611–2615
Casciola M, Costantino U, Merlini L, Andersen IGK, Andersen EK (1988) Preparation, structural characterization and conductivity of LiZr2(PO4)3. Solid State Ionics 26:229–235
Martinez-Juarez A, Rojo JM, Iglesias JE, Sanz J (1995) Reversible monoclinic-rhombohedral transformation in LiSn2(PO4)3 with NASICON-type structure. Chem Mater 7:1857–1862
Cruz AM, Ferreira EB, Rodrigues ACM (2009) Controlled crystallization and ionic conductivity of a nanostructured LiAlGePO4 glass-ceramic. J Non-Cryst Solids 355:2295–2301
Thokchom JS, Gupta N, Kumar B (2008) Superionic conductivity in a lithium aluminum germanium phosphate glass-ceramic. J Electrochem Soc 155:A915–A920
Inaguma Y, Liquan C, Itoh M, Nakamura T, Uchida T, Ikuta H, Wakihara M (1993) High ionic conductivity in lithium lanthanum titanate. Solid State Commun 86:689–693
Stramare S, Thangadurai V, Weppner W (2003) Lithium lanthanum titanates: a review. Chem Mater 15:3974–3990
Garcia-Martin S, Rojo JM, Tsukamoto H, Mora´n E, Alario-Franco MA (1999) Lithium-ion conductivity in the novel La1/3-xLi3xNbO3 solid solution with perovskite-related structure. Solid State Ionics 116:11–18
Kasper HM (1969) A new series of rare earth garnets Ln3M2Li3O12(M = Te, W). Inorg Chem 8:1000–1005
Geiger CA, Alekseev E, Lazic B, Fisch M, Armbruster T, Langner R, Fechtelkord M, Kim N, Pettke T, Weppner W (2011) Crystal chemistry and stability of “Li7La3Zr2O12” garnet: a fast lithium-ion conductor. Inorg Chem 50:1089–1097
Hong YP (1978) Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors. Mater Res Bull 13:117–124
Bruce PG, West AR (1980) Phase diagram of the LISICON, solid electrolyte system, Li4GeO4-Zn2GeO4. Mater Res Bull 15:379–385
Li Y, Han JT, Wang CA et al (2012) Optimizing Li+ conductivity in a garnet framework [J]. J Mater Chem 22:15357–15361
Zhang J, Zhao N, Zhang M, Li Y, Chu PK, Guo X, di Z, Wang X, Li H (2016) Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide[J]. Nano Energy 28:447–454
De B, Yadav A, Khan S et al (2017) A facile methodology for the development of printable and flexible all solid-state rechargeable battery[J]. ACS Appl Mater Interfaces 9:19870–19880
Zhang Z, Zhao Y, Chen S et al (2017) An advanced construction strategy of all-solid-state lithium batteries with excellent interfacial compatibility and ultralong cycle life[J]. J Mater Chem A 5
Liang Y, Lin Z, Qiu Y (2011) Fabrication and characterization of LATP/PAN composite fiber-based lithium-ion battery separator[J]. Electrochim Acta 56:6474–6480
Romero M, Faccio R, Vázquez S et al (2016) Enhancement of lithium conductivity and evidence of lithium dissociation for LLTO-PMMA nanocomposite electrolyte[J]. Mater Lett 172(jun.1):1–5
Chen YT, Jena A, Pang WK (2017) Voltammetric Enhancement of Li-Ion Conduction in Al-Doped Li7–xLa3Zr2O12 Solid Electrolyte[J]. J Phys Chem C
Zhang X, Liu T, Zhang S, Huang X, Xu B, Lin Y, Xu B, Li L, Nan CW, Shen Y (2017) Synergistic coupling between Li6.75La3Zr1.75Ta0.25O12 and poly (vinylidene fluoride) induces high ionic conductivity, mechanical strength, and thermal stability of solid composite electrolytes[J]. J Am Chem Soc 139(39):13779–13785
Ngai KS, Ramesh S, Ramesh K, Juan JC (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics 22:1259–1279
Liang X, Han D, Wang Y, Lan L, Mao J (2018) Preparation and performance study of a PVDF–LATP ceramic composite polymer electrolyte membrane for solid-state batteries[J]. RSC Adv 8(71):40498–40504
Chen RJ, Zhang YB, Liu T, Xu BQ, Lin YH, Nan CW, Shen Y (2017) addressing the interface issues in all-solid-state bulk-type lithium ion battery via an all-composite approach[J]. ACS Appl Mater Interfaces 9(11):9654–9661
Funding
This work is financially supported by the National Natural Science Foundation of China (Grants 51972023).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Liu, H., Li, J., Feng, W. et al. Strippable and flexible solid electrolyte membrane by coupling Li6.4La3Zr1.4Ta0.6O12 and insulating polyvinylidene fluoride for solid state lithium ion battery. Ionics 27, 3339–3346 (2021). https://doi.org/10.1007/s11581-021-04094-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-021-04094-x