Abstract
A sensitive and selective method for the simultaneous determination of trace gallium and indium in natural water samples using adsorptive stripping voltammetry at the Hg(Ag)FE electrode was established. The optimum analytical conditions include 0.2 mol L−1 acetate buffer (pH = 5.3) and 4 × 10−4 mol L−1 cupferron. The calibration graph was linear from 5 × 10−9 to 5 × 10−7 mol L−1 for the simultaneous presence of indium and gallium. The detection limits for preconcentration time of 50 s were 1.6 × 10−9 mol L−1 and 1.4 × 10−9 mol L−1 for gallium and indium, respectively. Selectivity of the method was determined by investigating the influence of numerous different foreign ions. The interferences of surfactants and humic substances were minimized by preliminary mixing with resin. Analytical results of natural water samples analysis showed that the proposed procedure is suitable for direct environmental water analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of the procedure for the simultaneous determination of Ga(III) and In(III) in environmental water samples results from an increased rate of production and utilization of these trace metals which are used in high-tech applications. In the literature, indium and gallium are often referred to as technology critical elements [1]. Both of these elements have similar, often desirable, valuable properties and therefore play a great role in the high technology industry, mainly in the production of semiconductors and electronic devices. Gallium enjoys vast application in optoelectronics (e.g., LED’s), telecommunication, aviation, and many commercial and household items such as alloys, computers, and DVD’s [2]. The biggest use of indium has been recorded in thin-film coatings in liquid crystal display screens (LCDs) used in computers and CD/DVD players, solar cells, electroluminescent lamps, and flat panel displays [3, 4]. In recent years, gallium and indium alloys have been used for 3D printing with liquid metals; these alloys allow one to create structures by piling drops on top of each other and to create specific shapes [5]. The broad use of these metals leads to their continuous introduction into the environment. As their use is similar, thus they often get into the environment from the same anthropogenic source. The possible environmental and (eco)toxicological effects due to the application of In and Ga in high-tech are still being investigated. There is little information about health effects to humans or animals due to exposure to indium and gallium compounds. However, some cases of poisoning with these metals are known. These metals are also carcinogenic agents [6, 7]. Oral ingestion of GaAs and InAs can cause serious symptoms such as gastrointestinal discomfort, vomiting, coma, and even death in the case of acute poisoning. The consequences of chronic poisoning with these compounds include anemia, leukopenia, skin cancer, and other internal cancers [8]. In the near future, the demand for indium and gallium is expected to continue to increase and thus the potential occupational exposure to the compounds of these metals attracts considerable attention [9]. Current toxicological data show that, with the exception of persons with heavy occupational exposure such as those employed in electronics industry, problems for the general population are rather unlikely [10]. As concerns environmental problems, In(III) and Ga(III) can cause soil and water contamination and be harmful to living beings. The soil-plant system is largely dependent on the quality of environmental waters; therefore, it is necessary to obtain information about the concentration of these elements in environmental water samples. So the demand for analytical techniques that would be able to perform quantification of these “technologically critical elements” is still growing.
In the literature data, various techniques for the simultaneous determination of Ga(III) and In(III) in environmental samples have been described. The vast majority of them were spectrometric methods such as electrothermal-atomization atomic absorption spectrometry [11, 12], inductively coupled plasma mass spectrometry [13], spectrophotometry [14, 15], and inductively coupled plasma optical emission spectrometry [16]. Because it is often advised to use two different procedures to verify the correctness of the performed determinations, preferably based on other techniques, the purpose of our work was to develop a procedure for the simultaneous determination of Ga(III) and In(III) using stripping voltammetry as the most commonly used electrochemical method because of its low cost, high sensitivity, and short measurement time. Amidst stripping voltammetric methods, we can distinguish anodic stripping voltammetry (ASV) and adsorptive stripping voltammetry (AdSV), where AdSV was preferable to obtain a lower detection limit. Up to now, multiple procedures that are characterized by different sensitivities for determining gallium and indium separately in environmental samples have been developed. The previously published adsorption voltammetry stripping methods for the determination of gallium(III) and indium(III) in environmental samples are collected in Table 1 for gallium and Table 2 for indium, respectively.
Our scientific group described in the literature an adsorption voltamerometric procedure for the simultaneous determination of Ga(III) and In(III) using a bismuth as a working electrode [32]. However, the detection limits obtained in this procedure were unsatisfactory. Therefore, the purpose of our further research was to develop a procedure for simultaneous determination of gallium and indium with lower detection limits. As can be seen in Tables 1 and 2 the lowest detection limits for both Ga(III) and In(III) determination procedures were obtained using a renewable mercury silver-based electrode (Hg(Ag)FE) as a working electrode. Therefore, this electrode was used in the presented work to develop a voltammetric procedure for the simultaneous determination of gallium and indium. The Hg(Ag)FE electrode is a viable alternative to the hanging mercury drop electrode (HMDE) because it ensures all merits of the mercury electrode such as a very low detection limit and additionally thanks to its construction significantly reducing toxicity, which is very important for laboratory environment. As a complexing agent, cupferron was chosen for our experiments, which is the most commonly used complexing agent in the adsorptive stripping voltammetry separate determinations of both gallium and indium (see Tables 1 and 2).
Experimental
Apparatus
All electrochemical measurements were performed using a μAutolab analyzer (Utrecht, The Netherlands). A three-electrode configuration consisted of an Hg(Ag)FE working electrode, a platinum wire counter electrode, and an Ag/AgCl reference electrode (in saturated NaCl). The solutions were stirred with a magnetic stirring bar. The Pt electrode and the Ag/AgCl electrode were prepared in our laboratory. The Hg(Ag)FE electrode was purchased from the MTM-ANKO Cracow, Poland (the mercury film area was 7 mm2). All experiments were carried out at room temperature.
The Hg(Ag)FE construction is based on pulling up the silver wire electrode base into the mercury chamber placed in the electrode corpus and then pushing it back outside the electrode corpus into the analyzed solution just before voltammetric measurement. In this way, the silver wire base is covered with a new mercury film and in this form, it is ready for use. After measurement, the electrode must be refreshed and this is obtained through pulling up the silver wire with mercury film inside the electrode. During this step, the silver wire crosses over special O-ring seals and a precise wipe out takes place [33].
Reagents and solutions
Standard solutions of 1 g L−1 Ga(III) and 1 g L−1 In(III) were obtained from Merck (Darmstadt, Germany) and Fluka (Buchs, Switzerland), respectively. The solutions of Ga(III) and In(III) of lower concentrations were prepared every day by dilution of the stock solution as required. Cupferron (N-nitrosophenylhydroxylamine ammonium salt) was obtained from Merck (Darmstadt, Germany). A solution of 1 × 10−2 mol L−1 of cupferron was prepared every day by dissolving 0.0155 g of the reagent in water in a 10-mL volumetric flask. The 1 mol L−1 acetate buffer (pH = 5.3) was prepared from Suprapur CH3COOH and NaOH obtained from Merck. Triton X-100, sodium dodecyl sulfate (SDS), and cetyltrimethylammonium bromide (CTAB) were purchased from Fluka (Buchs, Switzerland). Humic acid (HA) sodium salt was obtained from Aldrich. The river fulvic acid (FA) and natural organic matter (NOM) were obtained from the Suwannee River and purchased from the International Humic Substances Society. Rhamnolipids (biosurfactant) and Amberlite XAD-7 resin were obtained from Sigma (St. Louis, MO, USA). The resin was washed four times with triply distilled water and dried up at the temperature of 50 °C. All solutions were prepared using triply distilled water.
Bystrzyca river sample preparation
Natural river water samples from the Bystrzyca river were collected with polypropylene bottles and then filtered through 0.45 μm Millipore membrane filters. The samples were kept at the temperature of 6 °C and they were submitted to analysis without any pretreatment.
Standard procedure of voltammetric measurement
The standard voltammetric measurement was carried out in a solution containing 0.2 mol L−1 acetate buffer pH = 5.3 and 4 × 10−4 mol L−1 cupferron. The experiments were run from nondeaerated solutions with a volume of 10 mL.
The adsorptive voltammetric procedure consisted of the following main steps:
Deposition step: at two potentials successively following − 0.9 V for 20 s and − 0.7 V for 30 s, during that time the Ga(III)-cupferron and In(III)-cupferron complexes were accumulated simultaneously on the Hg(Ag)FE working electrode as a result of adsorption, under stirring solution.
Registration of the voltammogram: after a rest period of 5 s, a differential pulse stripping voltammogram was recorded under quiescent solution, while the potential was scanned from − 0.5 to − 1.2 V. Intensities of the obtained peaks were directly proportional to the concentration of Ga(III) and In(III) in the sample. The parameters of the differential pulse voltammetric measurement were as follows: scan rate 20 mVs−1 and pulse height 100 mV. The indium peak appeared at ~ − 0.65 V and the gallium peak appeared at ~ − 1.05 V.
After a single voltammetric measurement, the mercury film was refreshed as a result of inserting a silver wire on which the mercury film is formed into the center of the electrode. During that stage, the silver wires crossed with special O-rings and there was a precise wiping. Afterwards, before each subsequent measurement, the silver wire was ejected outside the electrode corpus through the mercury compartment and the mercury film was created [33].
Procedure of mixing with resin
In the case of analysis of real samples whose matrix is rich in organic substances such as surfactants and/or humic substances before the standard voltammetric measurement, it is advisable to remove the organic substances because they may interfere with the voltammetric measurement. To do this, the analysis of real samples with the voltammetric method should be preceded by mixing the analyzed sample with the Amberlite XAD-7 resin according to the following scheme. The analyzed sample solution, 4 mL of 1 mol L−1 acetate buffer pH = 5.3 and an adequate volume of triply distilled water, so that the final volume of the solution was 10 mL, should be added to a glass vial and 0.5 g of XAD-7 resin was inserted. Then, the prepared solution was stirred for 5 min using a magnetic stirring bar. During that time, the organic substances were adsorbed on the resin surfaces, while indium and gallium ions remained in the solution. After sedimentation of the resin, 5 mL of the solution was pipetted into the electrochemical cell. Next, 400 μL of 1 × 10−2 mol L−1 cupferron and 4.6 mL of triply distilled water were inserted consecutively into the electrochemical cell so that the desired concentrations were obtained (0.2 mol L−1 acetate buffer pH = 5.3 and 4 × 10−4 mol L−1 cupferron). Finally, the voltammetric measurement was performed as described in the previous chapter.
Results and discussion
In order to develop a new procedure for the simultaneous determination of In(III) and Ga(III) by AdSV method, the measurement conditions such as:
pH and concentration of the supporting electrolyte,
the concentration of the complexing agent,
the potential and time of accumulation of the indium and gallium complexes at the working electrode surface had to be chosen so as to obtain optimal signals concomitantly for both analyzed elements, considering the height, shape of the peaks, and their separation on the voltammogram. Previous studies showed that Ga(III) and In(III) form electrochemically active complexes with cupferron, which allow voltammetric determination of these elements with a low limit of detection [19, 25, 26, 32]. The choice of the working electrode was directed to provide the best sensitivity, and as we know, this can be obtained using mercury electrodes. However, it is also important that the applied electrode should not cause toxic effects. The Hg(Ag)FE electrode is much less toxic than the HMDE as a consequence of a lower amount of mercury, besides we are dealing with amalgam, not pure mercury as in the case of HMDE. Therefore, the current article presents the AdSV method applied for the simultaneous determination of In(III) and Ga(III) using cupferron as a complexing agent and a renewable mercury silver-based electrode as the working electrode.
Effect of pH and concentration of supporting electrolyte
In previously developed voltammetric procedures using the adsorptive metal accumulation at the working electrode surface in the form of a complex with cupferron, an acidic environment was used. Thus, based on available data concerning the determination procedures of In(III) and Ga(III) separately by the AdSV method as a supporting electrolyte, an acetate buffer was chosen as the most suitable for both Ga(III)-cupferron and In(III)-cupferron complex formation. Then, the focus was on choosing the appropriate pH of the acetate buffer. The measurements were carried out at the same concentration of each of the tested buffers, equal to 0.2 mol L−1 in the pH range from 3.6 to 5.6. The obtained results showing the effect of pH on the height of indium and gallium peaks are presented in Fig. 1. As can be seen, the highest signals for both indium and gallium were undoubtedly obtained for pH = 5.3 and above, so the acetate buffer pH 5.3 was chosen as the optimum one.
The effect of acetate buffer concentration on the indium and gallium peak currents was studied in the range from 0.1 to 0.4 mol L−1. It was observed that both peak currents were increasing with the increase of buffer concentration to 0.2 mol L−1 whereas at higher concentrations they remained unchanged. Consequently, as the supporting electrolyte, 0.2 mol L−1 acetate buffer pH 5.3 was used for further measurements.
Effect of cupferron concentration
Changing the concentration of the cupferron chelating agent can also have an enormous influence on the sensitivity of indium and gallium determination. The effect of cupferron concentration on the values of AdSV indium and gallium peak currents was studied in the range from 1 × 10−5 to 1 × 10−3 mol L−1. The indium peak appeared at the concentration of cupferron equal to 1 × 10−5 mol L−1 and increased with the increase of cupferron concentration up to 2 × 10−4 mol L−1. At concentrations of cupferron higher than 6 × 10−4 mol L−1, the peak of indium slightly decreased. Whereas, the gallium peak appeared at the concentration of cupferron 5 × 10−5 mol L−1 and increased upon increasing the concentration of cupferron to 4 × 10−4 mol L−1 and then it remained unchanged. So the concentration of cupferron equal to 4 × 10−4 mol L−1 was used as the optimum concentration for both determined elements. We also noticed that with increasing concentration of cupferron, both peaks were moving towards more negative potentials. The influence of cupferron concentration on the indium and gallium peak currents is presented in Fig. 2.
Conditions of accumulation potential and time of Ga(III)-cupferron and In(III)-cupferron
In order to assess the influence of accumulation potential directly on analytical results, the adsorptive stripping response of gallium and indium was studied in the solution containing 0.2 mol L−1 acetate buffer pH = 5.3, 4 × 10−4 mol L−1 cupferron, and 1 × 10−7 mol L−1 Ga(III) and In(III). The main goal was to obtain high and comparable gallium and indium signals at the same concentrations. The potential was examined in the range from − 1.0 to − 0.4 V with fixed deposition time of 50 s. It can be observed that upon changing accumulation potential of Ga(III)-cupferron and In(III)-cupferron towards more positive values, both obtained peaks were higher. Considering that the gallium peak increased only to the potential − 0.7 V and next it was stable, this potential was pre-selected. It was found that the accumulation potential did not influence the separation of the examined peaks in the entire examined range. Next the total accumulation time was tested in the range from 10 to 70 s. The values of the voltammetric peak currents increased almost linearly with increased total accumulation time up to 50 s both for gallium and indium and then they were constant.
As our goal was to obtain comparable gallium and indium signals at the same concentrations and at the accumulation potential − 0.7 V, the signal of In(III) was bigger than the signal of Ga(III); the next step was to investigate whether running the accumulation step at two different potentials could allow us to obtain comparable gallium and indium peak heights for the same concentrations. Therefore, apart from the accumulation potential of − 0.7 V, additional potential applied to the working electrode was added. The optimization of these parameters was carried out by changing the first potential (in the range from − 1.2 to − 0.7 V) for 20 s while the second potential was fixed − 0.7 V for 30 s. The almost equal heights of both signals were obtained at the combination of two potentials of − 0.9 V for 20 s and then − 0.7 V for 30 s, so these potentials were selected as the most optimum ones.
The calibration graph
Linear ranges and detection limits for the simultaneous determination of gallium and indium were evaluated under selected conditions: 0.2 mol L−1 acetic buffer (pH = 5.3), 4 × 10−4 mol L−1 cupferron, accumulation potential and time − 0.9 V for 20 s and − 0.7 V for 30 s. Linear calibration graphs were obtained in the concentration range of 5 × 10−9 to 5 × 10−7 mol L−1 for the simultaneous presence of Ga(III) and In(III) in the solution. They obeyed the following calibration equations: y = 567.3x + 15.4 (for Ga(III)) with the linear correlation coefficient r = 0.997, and y = 625.8x + 18.6 (for In(III)) with the linear correlation coefficient r = 0.998, where y and x are the peak current (μA) and concentration (nmol L−1), respectively. The detection limits estimated from three times the standard deviation of low Ga(III) and In(III) concentrations and accumulation time 50 s were about 1.6 × 10−9 mol L−1 and 1.4 × 10−9 mol L−1, respectively. The relative standard deviations (RSD) from six determinations at the concentrations 1 × 10−8 mol L−1 of Ga(III) and In(III) were 3.9% and 3.7%, respectively.
Selectivity
The selectivity of the Hg(Ag)FE electrode for the determination of gallium and indium was evaluated by introducing the concentrations of other metal ions as interfering species into solutions with constant concentration of Ga(III) and In(III) equal to 1 × 10−7 mol L−1. A tolerable limit was defined as the amount of foreign ions that produced an error not exceeding 5% in the peak currents of Ga(III) and In(III). The vast majority of ions in excess in relation to gallium and indium did not affect their voltammetric signal; however, in some cases, a different effect on the determined elements was observed, which is why the maximum tolerable concentrations of foreign ions for Ga(III) and In(III) are shown separately in Tables 3 and 4, respectively. The big advantage of the proposed procedure is that even a 20-fold excess of Cd(II) relative to In(III) does not affect the indium signal. This is particularly important in the case of Cd(II) which is serious interferents in anodic voltammetric determination of indium, because their reduction potentials are very close to reduction potentials of indium. In adsorptive voltammetric procedure using cupferron as a complexing agent, reduction potential of cadmium is at about − 0.58 V [34], while the indium reduction potential is at about − 0.65 V which ensures satisfactory separation of peaks at determined concentrations of these elements.
Interference of organic compounds
The proposed procedure was developed to analyze environmental water samples that naturally have an organic matrix. Consequently, in the course of this procedure, the interference generated by various organic compounds was precisely investigated and minimized. Among numerous organic substances commonly present in natural water are surface-active substances and humic substances.
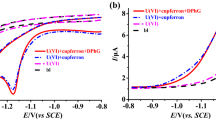
First, the influence of surface-active substances on analytical signals of gallium and indium in the proposed procedure was studied. Triton X-100-nonionic surfactant, SDS-anionic surfactant, CTAB-cationic surfactant, and Rhamnolipid-biosurfactant were selected to examine. Complete results of the impact of different types of surface-active substances using a standard procedure and the procedure with mixing with the resin are presented in Table 5. As can be seen, under the influence of even very small amounts of Triton X-100 (0.5 ppm), CTAB (0.5 ppm), and Rhamnolipid (1 ppm), the voltammetric signals of both gallium and indium were completely suppressed using a standard procedure. These substances clearly reduced both voltammetric signals. The anionic surfactant SDS did not show such a large interference because at a concentration of 5 ppm, it caused a decreased indium signal only by 60% and the gallium peak by 48%. Nevertheless, the preliminary mixing with the Amberlite XAD-7 resin significantly increased the allowable concentration in the analyzed sample of the surfactant, which did not exert any effect on the indium and gallium signals (for Triton X-100—1.5 ppm for In(III) and 2 ppm for Ga(III), for SDS and Rhamnolipids—5 ppm, for CTAB—2 ppm). The elimination of Triton X-100 interference was the least effective in relation to the indium signal leading to its reduction by almost 70% at a Triton X-100 concentration of 5 ppm.
The next step was to examine the impact of commercially available organic matter, such as HA, FA, and NOM on the voltammetric signals of gallium and indium. The measurements were performed similarly as for surface-active substances using the standard procedure and the preliminary mixing of the sample with the resin. The obtained results are presented in Table 6. As concerns indium, in the presence of humic substances, the observed interferences were not as big as in the presence of the surfactants using the standard procedure. However, it should be noted that the influence of humic substances on the gallium signal was more noticeable than on the indium signal. In the case of all examined humic substances, the elimination of their interferences by Amberlite resin was very effective (Table 6).
Application of the proposed procedures
In order to validate the proposed procedure, recovery tests were carried out by taking a fresh natural water sample from the Bystrzyca river (eastern areas of Poland). The voltammogram recorded for this sample did not show any signals of Ga(III) and In(III), which indicated that the concentrations of gallium and indium were below the detection limit of the proposed procedure. So the analyzed samples were spiked with Ga(III) and In(III) at different concentration levels and the contents of these elements were determined using the standard addition method. Three replicate determinations gave the average recovery values between 97.6 and 102.3% for In(III) with relative standard deviation between 4.7 and 5.5% and the average recovery values between 96.2 and 98.7% for Ga(III) with relative standard deviation between 4.4 and 5.3%. The typical voltammograms obtained during this analysis are presented in Fig. 3.
Conclusions
The renewable mercury film silver-based electrode (Hg(Ag)FE) and cupferron as an alternative for the simultaneous quantification of traces of Ga(III) and In(III) in one measurement by adsorptive stripping voltammetry were successfully proposed. The main advantage of the procedure is the use of a more environmentally friendly Hg(Ag)FE as the working electrode which is less toxic than the HMDE electrode and allows one to obtain comparable parameters. The application of the renewable mercury film silver-based electrode shortens the total time of measurements because in the case of this electrode, electrochemical cleaning is not necessary in contrast to film electrodes created electrochemically on glassy carbon such as PbFE or BiFE. Another advantage of the proposed procedure is the fact that no deaeration of the solution is necessary, which makes it easy to use under laboratory and field conditions. The application of Amberlite XAD-7 resin made it possible to elaborate a simple and fast voltammetric procedure in which interferences from surface-active compounds were minimized. To prove its practical applicability, the proposed procedure was successfully applied to the quantification of indium and gallium in environmental water samples. The above-described procedure looks promising and can be recommended for monitoring the water quality of environmental waters.
References
Cobelo-García A, Filella M (2017) Review article electroanalytical techniques for the quantification of technology-critical elements in environmental samples. Curr Opin Electrochem 3:78–90
Moskalyk RR (2003) Gallium: the backbone of the electronics industry. Miner Eng 16:921–929
Alfantazi AM, Moskalyk RR (2003) Processing of indium: a review. Miner Eng 16:687–694
Merian E, Anke M, Stoeppler M, Ihnat M (2004) Elements and their compounds in the environment, 2nd edn. Wiley-VCH Verlag GmbH&Co. KGaA, Weinheim
Daalkhaijav U, Yirmibesoglu OD, Walker S, Menguc Y (2018) Rheological modification of liquid metal for additive manufacturing of stretchable electronics. Adv Mater Technol 3:1700351
Chepesiuk R (1999) Y2K: the moment of truth. Environ Health Perspect 107:A252–A255
Fowler BA, Yamauchi H, Conner EA, Akkerman M (1993) Cancer risks for humans from exposure to the semiconductor metals. Scand J Work Environ Health 19:101–103
Betoulle S, Etienne JC, Vernet G (2002) Acute immunotoxicity of gallium to carp (Cyprinus carpio L.). Bull Environ Contam Toxicol 68:817–823
Tanaka A, Hirata M, Kiyohara Y, Nakano M, Omae K, Shiratani M, Koga K (2010) Review of pulmonary toxicity of indium compounds to animals and humans. Thin Solid Films 518:2934–2936
Hoet P, De Graef E, Swennen B, Seminck T, Yakoub Y, Deumer G, Haufroid V, Lison D (2012) Occupational exposure to indium: what does biomonitoring tell us? Toxicol Lett 213:122–128
Hayashibe Y, Kurosaki M, Takekawa F, Kuroda R (1989) Determination of traces of gallium and indium in ores by electrothermal-atomization atomic absorption spectrometry with matrix modification. Microchim Acta 98:163–171
Bermejo-Barrera P, Martinez-Alfonso N, Bermejo-Barrera A (2001) Separation of gallium and indium from ores matrix by sorption on Amberlite XAD-2 coated with PAN. Fresenius J Anal Chem 369:191–194
Orians KJ, Boyle EA (1993) Determination of picomolar concentrations of titanium, gallium and indium in sea water by inductively coupled plasma mass spectrometry following an 8-hydroxyquinoline chelating resin preconcentration. Anal Chim Acta 282:63–74
Zaki MTM, El-Didamony AM (1988) Determination of gallium and indium with haematoxylin in a micellar medium. Analyst 113:1277–1281
Singh VK, Agnihotri NK, Singh HB, Sharma RL (2001) Simultaneous determination of gallium(III) and indium(III) by derivative spectrophotometry. Talanta 55:799–806
Liu HM, Jiang JK, Lin YH (2012) Simultaneous determination of gallium(III) and indium(III) in urine and water samples with cloud point extraction and by inductively coupled plasma optical emission spectrometry. Anal Lett 45:2096–2107
Piech R (2009) Novel sensitive voltammetric detection of trace gallium(III) with presence of catechol using mercury film silver based electrode. Electroanalysis 21:1842–1847
Grabarczyk M, Wasąg J (2015) Determination of trace amounts of Ga(III) by adsorptive stripping voltammetry with in situ plated bismuth film electrode. Talanta 144:1091–1095
Grabarczyk M, Wardak C (2014) A new voltammetric strategy for sensitive and selective determination of gallium using cupferron as a complexing agent. J Environ Sci Health A 49:1142–1148
Li YH, Zhao QL, Huang MH (2005) Cathodic adsorptive voltammetry of the gallium-alizarin red S complex at a carbon paste electrode. Electroanalysis 17:343–347
Piech R (2011) Study on simultaneous measurements of trace gallium(III) and germanium(IV) by adsorptive stripping voltammetry using mercury film electrode. J Appl Electrochem 41:207–214
Qu L, Jin W (1993) Adsorption voltammetry of the gallium-morin system. Anal Chim Acta 274:65–70
Wang J, Zadeii JM (1986) Determination of traces of gallium based on stripping voltammetry with adsorptive accumulation. Anal Chim Acta 185:229–238
González MJG, Renedo OD, Lomillo MAA, Martínez MJA (2004) Determination of gallium by adsorptive stripping voltammetry. Talanta 62:457–462
Grabarczyk M, Wasąg J (2015) Adsorptive cathodic stripping voltammetric method for determination of gallium using an in situ plated lead film electrode. Electroanalysis 27:2596–2600
Grabarczyk M, Wasąg J (2016) Ultratrace determination of indium in natural water by adsorptive stripping voltammetry in the presence of cupferron as a complexing agent. J Electrochem Soc 163:H218–H222
Grabarczyk M, Wasąg J (2016) Application of a lead film electrode in adsorptive stripping voltammetry for the determination of indium trace in water samples. J Electrochem Soc 163:H465–H468
Benvidi A, Ardakani MM (2009) Subnanomolar determination of indium by adsorptive stripping differential pulse voltammetry using factorial design for optimization. Anal Lett 42:2430–2443
Farias PAM, Martin CML, Ohara AK, Gold JS (1994) Cathodic adsorptive stripping voltammetry of indium complexed with morin at a static mercury drop electrode. Anal Chim Acta 293:29–34
Paolicchi I, Renedo OD, Alonso Lomillo MA, Arcos Martínez MJ (2004) Application of an optimization procedure in adsorptive stripping voltammetry for the determination of trace contaminant metals in aqueous medium. Anal Chim Acta 511:223–229
Grabarczyk M, Wasąg J (2016) Adsorptive stripping voltammetry of In(III) in the presence of cupferron using an in situ plated bismuth film electrode. Anal Method 8:3605–3612
Grabarczyk M, Adamczyk M (2019) Simple, fast and cheap simultaneous quantification of Ga(III) and In(III) in environmental water samples. Int Agrophys 33:161–166
Bas B, Kowalski Z (2002) Preparation of silver surface for mercury film electrode of prolonged analytical application. Electroanalysis 14:1067–1071
Grabarczyk M, Koper A (2012) Direct determination of cadmium traces in natural water by adsorptive stripping voltammetry in the presence of cupferron as a chelating agent. Electroanalysis 24:33–36
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Grabarczyk, M., Adamczyk, M. & Wardak, C. Simultaneous AdSV determination of Ga and In on Hg(Ag)FE electrode by AdSV in presence of cupferron. Ionics 26, 1019–1027 (2020). https://doi.org/10.1007/s11581-019-03212-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-019-03212-0