Abstract
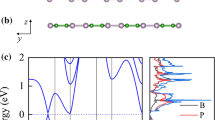
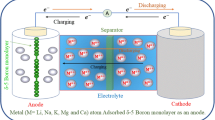
Three morphologies of two-dimension Boron with metallicity have been successfully synthetized by experiments. To access the potential of β12 borophene (□) and χ3 borophene monolayer (◇) as anode materials for lithium ion batteries, first-principles calculations based on density functional theory (DFT) are performed. Lithium atom is preferentially absorbed over the center of the hexagonal B atom hollow of β12 and χ3 borophene monolayer. The fully lithium storage phase of β12 and χ3 borophene monolayer corresponds to Li8B10 and Li8B16 with a theoretical specific capacity of 1983 and 1240 mA h g−1, respectively, much larger than other two-dimension materials. Interestingly, lithium ion diffusion on β12 borophene (□) monolayer is extremely fast with a low-energy barrier of 41 meV. Meanwhile, lithiated-borophene monolayer shows enhanced metallic conductivity during the whole lithiation process. Compared to the buckled borophene (△), the extremely enhanced lithium adsorption energy of β12 and χ3 phase with vacancies weakens lithium ion diffusion. Therefore, it is important to control the generation of vacancy in the buckled borophene (△) anode for lithium ion batteries. Borophene is a promising candidate with high capacity and high rate capability for anode material in lithium ion batteries.












Similar content being viewed by others
References
Wu L, Lee WH, Zhang J (2014) First Principles Study on the Electrochemical, Thermal and Mechanical Properties of LiCoO2 for Thin Film Rechargeable Battery. Materials Today: Proceedings 1(1):82–93. https://doi.org/10.1016/j.matpr.2014.09.017
Baker TA, Friend CM, Kaxiras E (2008) Chlorine interaction with defects on the Au(111) surface: A first-principles theoretical investigation. J Chem Phys 129(10):104702. https://doi.org/10.1063/1.2975329
Ong SP, Chevrier VL, Ceder G (2011) Comparison of small polaron migration and phase separation in olivine LiMnPO4 and LiFePO4 using hybrid density functional theory. Phys Rev B 83(7):540–545
Ouyang C, Shi S, Wang Z, Huang X, Chen L (2004) First-principles study of Li ion diffusion in LiFePO4. Phys Rev B 69(10):1–5
Jiang J, Ouyang C, Li H, Wang Z, Huang X, Chen L (2007) First-principles study on electronic structure of LiFePO4. Solid State Commun 143(3):144–148. https://doi.org/10.1016/j.ssc.2007.05.004
Li SN, Liu JB, Liu BX (2016) First principles study of nanostructured TiS2 electrodes for Na and Mg ion storage. J Power Sources 320:322–331. https://doi.org/10.1016/j.jpowsour.2016.04.122
Liu C, Neale ZG, Cao G (2016) Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater Today 19(2):109–123. https://doi.org/10.1016/j.mattod.2015.10.009
Rozier P, Tarascon JM (2015) Review—Li-Rich Layered Oxide Cathodes for Next-Generation Li-Ion Batteries: Chances and Challenges. J Electrochem Soc 162(14):A2490–A2499. https://doi.org/10.1149/2.0111514jes
Goodenough JB (2015) Energy storage materials: A perspective. Energy Storage Materials 1:158–161. https://doi.org/10.1016/j.ensm.2015.07.001
Zhang S, Du H, He J, Huang C, Liu H, Cui G, Li Y (2016) High Temperature Carbonized Grass as a High Performance Sodium Ion Battery Anode. ACS Appl Mater Interfaces 9(1):391–397. https://doi.org/10.1021/acsami.6b12542
Sun Q, Dai Y, Ma Y, Jing T, Wei W, Huang B (2016) Ab Initio Prediction and Characterization of Mo2 C Monolayer as Anodes for Lithium-Ion and Sodium-Ion Batteries. J Phys Chem Lett 7(6):937–943
Zhou X, Wang Z, Chen W, Ma L, Chen D, Lee JY (2014) Facile synthesis and electrochemical properties of two dimensional layered MoS2/graphene composite for reversible lithium storage. J Power Sources 251:264–268. https://doi.org/10.1016/j.jpowsour.2013.11.060
Liu Y, Zhao Y, Jiao L, Chen J (2014) A graphene-like MoS2/graphene nanocomposite as a highperformance anode for lithium ion batteries. J Mater Chem A 2(32):13109–13115. https://doi.org/10.1039/C4TA01644K
Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, Ruoff RS (2010) Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv Mater 22(35):3906–3924. https://doi.org/10.1002/adma.201001068
Xu Z, Lv X, Li J, Chen J, Liu Q (2016) A promising anode material for sodium-ion battery with high capacity and high diffusion ability: graphyne and graphdiyne. RSC Adv 6(30):25594–25600. https://doi.org/10.1039/C6RA01870J
Li Y, Xu L, Liu H, Li Y (2014) Graphdiyne and graphyne: from theoretical predictions to practical construction. Chem Soc Rev 43(8):2572–2586. https://doi.org/10.1039/c3cs60388a
Ivanovskii AL (2013) Graphynes and graphdyines. Prog Solid State Chem 41(1-2):1–19. https://doi.org/10.1016/j.progsolidstchem.2012.12.001
Li W, Yang Y, Zhang G, Zhang YW (2015) Ultrafast and Directional Diffusion of Lithium in Phosphorene for High-Performance Lithium-Ion Battery. Nano Lett 15(3):1691–1697. https://doi.org/10.1021/nl504336h
Guo GC, Wei XL, Wang D, Luo Y, Liu LM (2015) Pristine and defect-containing phosphorene as promising anode materials for rechargeable Li batteries. J Mater Chem A 3(21):11246–11252
Cai Y, Ke Q, Zhang G, Feng YP, Shenoy VB, Zhang Y-W (2015) Giant phononic anisotropy and unusual anharmonicity of phosphorene: interlayer coupling and strain engineering. Adv Funct Mater 25(15):2230–2236. https://doi.org/10.1002/adfm.201404294
Najmaei S, Yuan J, Zhang J, Ajayan P, Lou J (2015) Synthesis and defect investigation of two dimensional molybdenum disulfide atomic layers. Acc Chem Res 48(1):31–40
Zhou X, Wan LJ, Guo YG (2013) Synthesis of MoS2 nanosheet–graphene nanosheet hybrid materials for stable lithium storage. Chem Commun (Camb) 49(18):1838–1840. https://doi.org/10.1039/c3cc38780a
Kong D, Wang H, Cha JJ, Pasta M, Koski KJ, Yao J, Cui Y (2013) Synthesis of MoS2and MoSe2 films with vertically aligned layers. Nano Lett 13(3):1341–1347. https://doi.org/10.1021/nl400258t
Zhang C, Wang Z, Guo Z, Lou XW (2012) Synthesis of MoS2–C one-dimensional nanostructures with improved lithium storage properties. ACS Appl Mater Interfaces 4(7):3765–3768. https://doi.org/10.1021/am301055z
Lee YH, Zhang XQ, Zhang W, Chang MT, Lin CT, Chang KD, Yu YC, Wang JT, Chang CS, Li LJ, Lin TW (2012) Synthesis of large-area mos2 atomic layers with chemical vapor deposition. Adv Mater 24(17):2320–2325. https://doi.org/10.1002/adma.201104798
Xu C, Wang L, Liu Z, Chen L, Guo J, Kang N, Ma XL, Cheng HM, Ren W (2015) Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat Mater 14(11):1135–1141. https://doi.org/10.1038/nmat4374
Zhang H-J, Wang K-X, Wu X-Y, Jiang Y-M, Zhai Y-B, Wang C, Wei X, Chen J-S (2014) MoO2/Mo2C heteronanotubes function as high-performance Li-ion battery electrode. Adv Funct Mater 24(22):3399–3404. https://doi.org/10.1002/adfm.201303856
Mannix AJ, Zhou XF, Kiraly B, Wood JD, Alducin D, Myers BD et al (2015) Synthesis of borophenes: anisotropic two-dimensional boron polymorphs. Science 350(6267):1513–1516
Feng B, Zhang J, Zhong Q, Li W, Li S, Li H, Cheng P, Meng S, Chen L, Wu K (2016) Experimental realization of two-dimensional boron sheets. Nat Chem 8(6):563–568. https://doi.org/10.1038/nchem.2491
Penev ES, Kutana A, Yakobson BI (2016) Can two-dimensional boron superconduct? Nano Lett 16(4):2522–2526. https://doi.org/10.1021/acs.nanolett.6b00070
Jiang HR, Lu Z, Wu MC, Ciucci F, Zhao TS (2016) Borophene: a promising anode material offering high specific capacity and high rate capability for lithium-ion batteries. Nano Energy 23:97–104. https://doi.org/10.1016/j.nanoen.2016.03.013
Jena NK, Araujo RB, Shukla V, Ahuja R (2017) Borophane as a bench-mate of graphene: a potential 2d material for anode of li and na-ion batteries. ACS Appl Mater Interfaces 9(19):16148–16158
Mortazavi B, Dianat A, Rahaman O, Cuniberti G, Rabczu T (2016) Borophene as an anode material for Ca, Mg, Na or Li ion storage: A first-principle study. J Power Sources 329:456–461. https://doi.org/10.1016/j.jpowsour.2016.08.109
Mortazavia B, Rahamana O, Ahzib S, Rabczuk T (2017) Flat borophene films as anode materials for Mg, Na or Li-ion batteries with ultra high capacities: a first-principles study. Appl Materials Today 8:60–67. https://doi.org/10.1016/j.apmt.2017.04.010
Zhang Y, Wu ZF, Gao PF, Zhang S, Wen YH (2016) Could borophene be used as a promising anode material for high-performance li ion battery?. ACS Appl Mater Interfaces 8(34):22175–22181
Zhang X, Hu J, Cheng Y, Yang HY, Yao Y, Yang SA (2016) Borophene as an extremely high capacity electrode material for li-ion and na-ion batteries. Nanoscale 8(33):15340-15347
Perdew ARJP, Csonka GI (2008) Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys Rev Lett 100(13):136406. https://doi.org/10.1103/PhysRevLett.100.136406
Laasonen K, Car R, Lee C, Vanderbilt D (1991) Implementation of ultrasoft pseudopotentials in ab initio molecular dynamics. Phys Rev B Condens Matter 43(8):6796–6799
Monkhorst JDPHJ (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188–5192. https://doi.org/10.1103/PhysRevB.13.5188
H.N. Kunihiro Nobuhara, Masafumi Nose, Shinji Nakanishi, Hideki IBA, J Power Sources, 243 (2013) 585–587
Lv X, Xu Z, Li J, Chen J, Liu Q (2016) Investigation of fluorine adsorption on nitrogen doped MgAl 2 O 4 surface by first-principles. Appl Surf Sci 376:97–104. https://doi.org/10.1016/j.apsusc.2016.03.108
Liu M, Kutana A, Liu Y, Yakobson BI (2014) First-principles studies of li nucleation on graphene. J Physical Chemistry Letters 5(7):1225–1229. https://doi.org/10.1021/jz500199d
Fan X, Zheng WT, Kuo JL, Singh DJ (2013) Adsorption of single Li and the formation of small Li clusters on graphene for the anode of lithium-ion batteries. ACS Appl Mater Interfaces 5(16):7793–7797. https://doi.org/10.1021/am401548c
Li Q-F, Duan C-G, Wan XG, Kuo J-L (2015) Theoretical prediction of anode materials in Li-ion batteries on layered black and blue phosphorus. J Phys Chem C 119(16):8662–8670. https://doi.org/10.1021/jp512411g
Tritsaris GA, Kaxiras E, Meng S, Wang E (2013) Adsorption and diffusion of lithium on layered silicon for Li-ion storage. Nano Lett 13(5):2258–2263. https://doi.org/10.1021/nl400830u
Govind N, Petersen M, Fitzgerald G, King-Smith D, Andzelm J (2003) A generalized synchronous transit method for transition state location. Comput Mater Sci 28(2):250–258. https://doi.org/10.1016/S0927-0256(03)00111-3
Zhang R, Wu X, Yang J (2016) Blockage of ultrafast and directional diffusion of li atoms on phosphorene with intrinsic defects. Nanoscale 8(7):4001–4006
Komesu T, Le D, Zhang X, Ma Q, Schwier EF, Kojima Y, Zheng M, Iwasawa H, Shimada K, Taniguchi M, Bartels L, Rahman TS, Dowben PA (2014) Occupied and unoccupied electronic structure of Na doped MoS2(0001). 105(24):241602–1–241602–4
Sascha Thinius MMI, Heitjans P, Bredow T (2014) J Phys Chem C 118:2273–2280
Yazami RT, Reversible PA (1983) A reversible graphite-lithium negative electrode for electrochemical generators. J Power Sources 9(3):365–371. https://doi.org/10.1016/0378-7753(83)87040-2
Jishi RA, Dresselhaus MS (1992) Superconductivity in graphite intercalation compounds. Phys Rev B 45(21):12465–12469. https://doi.org/10.1103/PhysRevB.45.12465
Zhang H, Xia Y, Bu H, Wang X, Zhang M, Luo Y, Zhao M (2013) J Appl Phys 113:044309
Xiao J, Choi D, Cosimbescu L, Koech P, Liu J, Lemmon JP (2010) Exfoliated MoS2 nanocomposite as an anode material for lithium ion batteries. Chem Mater 22(16):4522–4524. https://doi.org/10.1021/cm101254j
Wan W, Zhang Q, Cui Y, Wang E (2010) J Physics. Condensed matter : an Institute of Physics journal 22(41):415501. https://doi.org/10.1088/0953-8984/22/41/415501
Chen X, He J, Srivastava D, Li J (2012) Electrochemical cycling reversibility of LiMoS2 using first-principles calculations. 100(26):263901–1–263901–4
Yafei Li DW, Zhou Z, Cabrera CR, Chen Z (2012) J Phys Chem Lett 3:2221–2227
Er D, Li J, Naguib M, Gogotsi Y, Shenoy VB (2014) ACS Appl Mater Interfaces 6(14):11173–11179. https://doi.org/10.1021/am501144q
Yi T-F, Xie Y, Zhu Y-R, Zhu R-S, Shen H (2013) Structural and thermodynamic stability of Li4Ti5O12 anode material for lithium-ion battery. J Power Sources 222:448–454. https://doi.org/10.1016/j.jpowsour.2012.09.020
Zhao B, Ran R, Liu M, Shao Z (2015) A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: the latest advancements and future perspectives. Materials Science and Engineering: R: Reports 98:1–71. https://doi.org/10.1016/j.mser.2015.10.001
Zhang W-J (2011) A review of the electrochemical performance of alloy anodes for lithium-ion batteries. J Power Sources 196(1):13–24. https://doi.org/10.1016/j.jpowsour.2010.07.020
Acknowledgements
This work was supported by the Kunming University of Science and Technology Analysis Test Fund (2017T20170001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Zhang, L. & Xu, L. Theoretical prediction of borophene monolayer as anode materials for high-performance lithium-ion batteries. Ionics 24, 1603–1615 (2018). https://doi.org/10.1007/s11581-017-2345-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2345-x