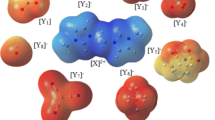
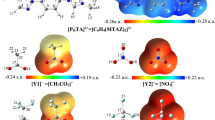
Abstract
In the present work, molecular engineering of the physicochemical characteristics of ion pairing in 1-methyl-4-phenyl 1,2,4 triazolium-based ionic liquids [PhMTZ][X] (X1–10 = CH3CO2 −, Cl−, NO3 −, CF3CO2 −, BF4 −, ClO4 −, N(CN)2 −, PF6 −, NTf2 −, and C(CN)3 −) are explored using at M06-2X/6–311++G(d,p) level. The binding Gibbs free energy of ion pairs are reevaluated using ab initio MP2 method and dispersion corrected M06-2X-D3, B2PLYP, B2PLYP-D, and mPW2PLYP-D functionals. Comparison of Gibbs free bottom electrodes (BEs) calculated by B2PLYP and B2PLYP-D functionals reveals that the contribution of dispersion energy to the total BEs vary from 9% for X1 to 17% for X = 10. Besides, the range of the dispersion contribution estimated by M06-2X-D3 functional is found to be 0.6% for X2 to 5% for X3. The Gibbs free BEs in solvent media, Gibbs free energy and enthalpy of formation, electrochemical windows, anodic and cathodic stability, volumetric and electron density properties, charge transfer values, and electrostatic maps are evaluated.











Similar content being viewed by others
References
Shamsi SH, Danielson ND (2007) Utility of ionic liquids in analytical separations. J Sep Sci 30:1729–1750
Earle MJ, Esperanca J, Gilea MA, Lopes JNC, Rebelo LPN, Magee JW, Seddon KR, Widegren JA (2006) The distillation and volatility of ionic liquids. Nature 439:831–834
Wasserscheid P (2006) Chemistry: Volatile times for ionic liquids. Nature 439:797–797
Endres F, Zein El Abedin S (2006) Air and water stable ionic liquids in physical chemistry. Phys Chem Chem Phys 8:2101–2116
Wassercheid P, Keim W (2000) Ionic liquids—new “solutions” for transition metal catalysis. Angew Chem Int Ed 39:3772–3789
Klapötke TM, Petermayer CD, Piercey GJ, Stierstorfer J (2012) The 1,3-bis(nitroimide)-1,2,3-triazolate anion, the N-nitroimide moiety, and the strategy of alternating positive and negative charges in the design of energetic materials. J Am Chem Soc 134:20827–20836
Joo YH, Shreeve JM (2009) Energetic mono-, di-, and trisubstituted nitroiminotetrazoles. Angew Chem 48:572–575
Thottempudi V, Shreeve JM (2011) Synthesis and promising properties of a new family of high-density energetic salts of 5-nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,50-bis(trinitromethyl)-3,30-azo-1H-1,2,4-triazole. J Am Chem Soc 133:19982–19992
Joo YH, Shreeve JM (2010) High-density energetic mono- or bis(oxy)-5-nitroiminotetrazoles. Angew Chem Int Ed 49:7320–7323
Joo YH, Shreeve JM (2010) Nitroimino-tetrazolates and oxy-nitroimino-tetrazolates. J Am Chem Soc 132:15081–15090
Klapötke TM, Stierstorfer J (2008) The CN7 − anion. J Am Chem Soc 131:1122–1134
Wang R, Xu H, Guo Y, Sa R, Shreeve JM (2010) Bis[3-(5-nitroimino-1,2,4-triazolate)]-based energetic salts: synthesis and promising properties of a new family of high-density insensitive materials. J Am Chem Soc 132:11904–11905
Zhang Q, Shreeve JM (2013) Ionic liquid propellants: Future fuels for space propulsion. J M Chem Eur 19:15446–15451
Sebastiao E, Cook C, Hu A, Murugesu M (2014) Recent developments in the field of energetic ionic liquids. J Mater Chem A 2:8153–8173
Zhang Q, Shreeve JM (2014) Energetic ionic liquids as explosives and propellant fuels: a new journey of ionic liquid chemistry. Chem Rev 114:10527–10574
Izgorodina EI, Seeger ZL, Scarborough DLA, Tan SYS (2017) Quantum chemical methods for the prediction of energetic, physical, and spectroscopic properties of ionic liquids. Chem Rev 117(10):6696–6754 and references therein
Kermanioryani M, Mutalib MIA, Dong Y, Lethesh KC, Ben Ghanem OBO, Kurnia KA, Leveque JM (2016) Physicochemical properties of new imidazolium-based ionic liquids containing aromatic group. J Chem Eng Data 61:2020–2026
Ben Ghanem O, Mutalib MA, Lévêque JM, Gonfa G, Kait CF, El-Harbawi M (2015) Studies on the physicochemical properties of ionic liquids based on 1-octyl-3-methylimidazolium amino acids. J Chem Eng Data 60:1756–1763
Fang DW, Yan Q, Li D, Xia LX, Zang SL (2014) Estimation of physicochemical properties of 1-alkyl-3-methylimidazolium glutamate. J Chem Therm 79:12–18
Wei Y, Jin Y, Wu ZJ, Yang Y, Zhang QG, Kang ZH (2013) Synthesis and physicochemical properties of amino acid ionic liquid 1-butyl-3-methylimidazolium aspartate and binary mixture with methanol. J Chem Eng Data 58:349–356
Giełdoń A, Bobrowski M, Bielicka-Giełdoń A, Czaplewski C (2016) Theoretical calculation of the physico-chemical properties of 1-butyl-4-methylpyridinium based ionic liquids. J Mol Liq 225:467–474
Ounissi A, Benguerba Y, Ouddai N (2016) Theoretical investigation on structural and physicochemical properties of some ionic liquids. Computational and Theoretical Chemistry 1092:68–73
Bahadur I, Kgomotso M, Ebenso EE, Redhi G (2016) Influence of temperature on molecular interactions of imidazolium-based ionic liquids with acetophenone: thermodynamic properties and quantum chemical studies. RSC Adv 6:104708–104723
Zhang Q, Lan Y, Liu H, Zhang X, Zhang X, Wei Y (2016) Estimation and structural effect on physicochemical properties of alkylimidazolium-based ionic liquids with different anions. J Chem Eng Data 61:2002–2012
Liu X, Su Z, Ji W, Chen S, Wei Q, Xie G, Yang X, Gao S (2014) Structure, physicochemical properties, and density functional theory calculation of high-energy-density materials constructed with intermolecular interaction: nitro group charge determines sensitivity. J Phys Chem C 118:23487–23498
Dong LL, He L, Liu HY, Tao GH, Nie FD, Huang M, Hu CW (2013) Nitrogen-rich energetic ionic liquids based on the N, N-bis (1H-tetrazol-5-yl) amine anion–syntheses, structures, and properties. Eur J Inorg Chem 28:5009–5019
De La Hoz AT, Brauer UG, Miller KM (2014) Physicochemical and thermal properties for a series of 1-alkyl-4-methyl-1, 2, 4-triazolium bis (trifluoromethylsulfonyl) imide ionic liquids. J Phys Chem B 118:9944–9951
Wu JT, Zhang JG, Yin X, Wu L (2016) Energetic salts based on 3-hydrazino-4-amino-1, 2, 4-triazole (HATr): synthesis and properties. New J Chem 40:5414–5419
Brauer UG, Andreah T, Miller KM (2015) The effect of counteranion on the physicochemical and thermal properties of 4-methyl-1-propyl-1,2,4-triazolium ionic liquids. J Mol Liq 210:286–292
Singh D, Gardas RL (2016) Influence of cation size on the Ionicity, fluidity and physiochemical properties of 1,2,4-triazolium based ionic liquids. J Phys Chem B 120:4834–4842
Watkins JD, Roth EA, Lartey M, Albenze E, Zhong M, Luebke DR, Nulwala HB (2015) Ionic liquid regioisomers: structure effect on the thermal and physical properties. New J Chem 39:1563–1566
Lartey M, Meyer-Ilse J, Watkins JD, Roth EA, Bowser S, Kusuma VA, Damodaran K, Zhou X, Haranczyk M, Albenze E, Luebke DR, Hopkinson D, Kortright JB, Nulwala HB (2015) Branched isomeric 1,2,3-triazolium-based ionic liquids: new insight into structure–property relationships. Phys Chem Chem Phys 17:29834–29843
Yan F, Lartey M, Jariwala K, Bowser S, Damodaran K, Albenze E, Luebke DR, Nulwala HB, Smit B, Haranczyk M (2014) Toward a materials genome approach for ionic liquids: synthesis guided by ab initio property maps. J Phys Chem B 118(47):13609–13620
Osada I, de Vries H, Scrosati B, Passerini S (2016) Ionic-liquid-based polymer electrolytes for battery applications. Angew Chem Int Ed 55:500–513
MacFarlane DR, Tachikawa N, Forsyth M, Pringle JM, Howlett PC, Elliott GD, Angell CA (2014) Energy applications of ionic liquids. Energy Environmental Scienc 7:232–250
MacFarlane DR, Forsyth M, Howlett PC, Kar M, Passerini S, Pringle JM, Zhang S (2016) Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nature Reviews Materials 11:1–15
Karakulina A, Gopakumar A, Akçok I, Roulier BL, LaGrange T, Katsyuba SA, Dyson PJ (2016) Rhodium nanoparticle–Lewis acidic ionic liquid catalyst for the chemoselective reduction of Heteroarenes. Angew Chem 128:300–304
Jie H, Cui X, Zhang Y, Feng T, Li X, Lin R, Xu L (2016) Transesterification of methyl acetate with isobutanol in a reactive and extractive distillation column with ionic liquid as catalyst and molecular liquid as entrainer. Ind Eng Chem Res 55:404–419
Sezginel KB, Keskin S, Uzun A (2016) Tuning the gas separation performance of CuBTC by ionic liquid incorporation. Langmuir 32:1139–1147
Cowan MG, Masuda M, McDanel WM, Kohno Y, Gin DL, Noble RD (2016) Phosphonium-based poly (ionic liquid) membranes: the effect of cation alkyl chain length on light gas separation properties and ionic conductivity. J Membrane Sci 498:408–413
Smigla M, Pringle JM, Lu X, Han L, Zhang S, Gao H, MacFarlane DR, Rogers RD (2014) Ionic liquids for energy, materials, and medicine. Chem Commun 50:9228–9250
Chatzimitakos T, Binellas C, Maidatsi K, Stalikas C (2016) Magnetic ionic liquid in stirring-assisted drop-breakup microextraction: proof-of-concept extraction of phenolic endocrine disrupters and acidic pharmaceuticals. Anal Chim Acta 910:53–59
An JH, Jin F, Kim HS, Ryu HC, Kim JS, Kim HM, Jung K (2016) Application of ionic liquid to polymorphic transformation of anti-viral/HIV drug adefovir dipivoxil. Arch Pharm Res 39:646–659
Wojnarowska Z, Knapik J, Rams-Baron M, Jedrzejowska A, Paczkowska M, Krause A, Paluch M (2016) Amorphous protic ionic systems as promising active pharmaceutical ingredients: The case of the Sumatriptan succinate drug. Mol Pharm 13:1111–1122
Meyer D, Strassner T (2011) 1,2,4-triazole-based tunable aryl/alkyl ionic liquids. J Org Chem 76:305–308
Daily LA, Miller KM (2013) Correlating structure with thermal properties for a series of 1-alkyl-4-methyl-1, 2, 4-triazolium ionic liquids. J Org Chem 78:4196–4201
Silvester DS, Compton RG (2006) Electrochemistry in room temperature ionic liquids: a review and some possible applications. Phys Chem 220:1247–1274
Buzzeo MC, Evans RG, Compton RG (2004) Non-haloaluminate room-temperature ionic liquids in electrochemistry—a review. Chem Phys Chem 5:1106–1120
Koch VR, Dominey LA, Nanjundiah C (1996) The intrinsic anodic stability of several anions comprising solvent-free ionic liquids. J Electrochem Soc 143:798–803
Kroon MC, Buijs W, Peters CJ, Witkamp GJ (2006) Decomposition of ionic liquids in electrochemical processing. Green Chem 8:241–245
Yüce AO, Mert BD, Kardas G, Yazıcı B (2014) Electrochemical and quantum chemical studies of 2-amino-4-methyl-thiazole as corrosion inhibitor for mild steel in HCl solution. Corros Sci 183:310–316
Ong SP, Andreussi O, Wu Y, Marzari N, Ceder G (2011) Electrochemical windows of room-temperature ionic liquids from molecular dynamics and density functional theory calculations. Chem Mater 23:2979–2986
Buijs W, Witkamp G-J, Kroon MC (2012) Correlation between quantumchemically calculated lumo energies and the electrochemical window of ionic liquids with reduction-resistant anions. Int J Electrochem 2012:589050–589056
Galinski M, Lewandowski A, Stepniak I (2006) Ionic liquids as electrolytes. Electrochim Acta 51:5567–5580
Hapiot P, Lagrost C (2008) Electrochemical reactivity in room-temperature ionic liquids. Chem Rev 108:2238–2264
Zhang Y, Shi C, Brennecke JF, Maginn EJ (2014) Refined method for predicting electrochemical windows of ionic liquids and experimental validation studies. J Phys Chem B 118:6250–6255
Mousavi MP, Kashefolgheta S, Stein A, Bühlmann P (2016) Electrochemical stability of quaternary ammonium cations: an experimental and computational study. J Electrochem Soc 2016(163):H74–H80
Kazemiabnavi S, Zhang Z, Thornton K, Banerjee S (2016) Electrochemical stability window of imidazolium-based ionic liquids as electrolytes for lithium batteries. J Phys Chem B 120:5691–5702
Wang H, Gu S, Bai Y, Chen S, Zhu N, Wu C, Wu F (2015) Anion-effects on electrochemical properties of ionic liquid electrolytes for rechargeable aluminum batteries. J Mater Chem A 3:22677–22686
Wu F, Zhu N, Bai Y, Liu L, Zhou H, Wu C (2016) Highly safe ionic liquid electrolytes for sodium-ion battery: wide electrochemical window and good thermal stability. ACS Appl Mater Interfaces 8:21381–21386
O’Mahony A, Silvester DS, Aldous L, Hardacre C, Compton RG (2008) Effect of water on the electrochemical window and potential limits of room-temperature ionic liquids. J Chem Eng Data 53:2884–2891
Abdul-Sada AK, Greenway AM, Hitchcock PB, Mohammed TJ, Seddon KR, Zora JA (1986) Upon the structure of room temperature halogenoaluminate ionic liquids. J Chem Soc Chem Commun 24:1753–1754
Wulf A, Fumino K, Ludwig R (2010) Spectroscopic evidence for an enhanced anion–cation interaction from hydrogen bonding in pure imidazolium ionic liquids. Angew Chem Int Ed 49:449–453
Katsyuba S, Zvereva EE, Vidis A, Dyson PJ (2007) Application of density functional theory and vibrational spectroscopy toward the rational design of ionic liquids. J Phys Chem B 111:352–370
Fumino K, Wulf A, Ludwig R (2008) The cation–anion interaction in ionic liquids probed by far-infrared spectroscopy. Angew Chem Int Ed 47:3830–3834
Wulf A, Fumino K, Ludwig R (2010) Spectroscopic evidence for an enhanced anion-cation interaction due to hydrogen bonding in pure imidazolium ionic liquids. Angew Chem Int Ed 49:449–453
Wakai C, Oleinikova A, Ott M, Weingartner H (2005) How polar are ionic liquids? Determination of the static dielectric constant of an imidazolium-based ionic liquid by microwave dielectric spectroscopy. J Phys Chem B 109:17028–17030
Schroder C, Hunger J, Stoppa A, Buchner R, Steinhauser O (2008) On the collective network of ionic liquid/water mixtures. II Decomposition and interpretation of dielectric spectra. J Chem Phys 129:184501–184510
Men S, Lovelock KRJ, Licence P (2011) X-ray photoelectron spectroscopy of pyrrolidinium-based ionic liquids: cation–anion interactions and a comparison to imidazolium-based analogues. Phys Chem Chem Phys 13:15244–15255
Fumino K, Fossog V, Wittler K, Hempelmann R, Ludwig R (2013) Dissecting anion–cation interaction energies in protic ionic liquids. Angew Chem Int Ed 52:2368–2372
Blundell RK, Licence P (2014) Tuning cation–anion interactions in ionic liquids by changing the conformational flexibility of the cation. Chem Commun 50:12080–12083
Lahiri A, Li G, Olschewski M, Endres F (2016) Influence of polar organic solvents in an ionic liquid containing lithium bis (fluorosulfonyl) amide: effect on the cation-anion interaction, Lithium ion Battery Performance, and Solid Electrolyte Interphase. ACS Appl Mater Interfaces 8:34143–34150
Mao Y, Damodaran K (2014) Ionization dynamics in ionic liquids probed via self-diffusion coefficient measurements. Chem Phys 440(31):87–93
Allen JJ, Bowser SR, Krishnan Damodaran K (2014) Molecular interactions in the ionic liquid emim acetate and water binary mixtures probed via NMR spin relaxation and exchange spectroscopy. Phys Chem Chem Phys 16:8078–8085
Allen JJ, Schneider Y, Kail BW, Luebke DR, Nulwala H, Damodaran K (2013) Nuclear spin relaxation and molecular interactions of a novel triazolium-based ionic liquid, 117: 3877–3883
Berg RW, Riisager A, Van Buu ON, Fehrmann R, Harris P, Tomaszowska AA, Seddon KR (2009) Crystal structure, vibrational spectroscopy and ab initio density functional theory calculations on the ionic liquid forming 1,1,3,3-tetramethylguanidinium bis{(trifluoromethyl) sulfonyl}amide. J Phys Chem B 113:8878–8886
Katsyuba SA, Griaznova TP, Vidis A, Dyson PJ (2009) Structural studies of the ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate in dichloromethane using a combined DFT-NMR spectroscopic approach. J Phys Chem B 113:5046–5051
Xiao D, Hines LG, Holtz MW, Song KY, Bartsch RA, Quitevis EL (2010) Effect of cation symmetry on the low-frequency spectra of imidazolium ionic liquids: OKE and Raman spectroscopic measurements and DFT calculations. Chem Phys Lett 497:37–52
Zhang S, Qi X, Ma X, Lu L, Zhang Q, Deng Y (2012) Investigation of cation–anion interaction in 1-(2-hydroxyethyl)-3-methylimidazolium-based ion pairs by density functional theory calculations and experiments. J Phys Org Chem 25:248–257
Fumino K, Reimann S, Ludwig R (2014) Probing molecular interaction in ionic liquids by low frequency spectroscopy: Coulomb energy, hydrogen bonding and dispersion forces. Phys Chem Chem Phys 16:21903–21929
Fumino K, Ludwig R (2014) Analyzing the interaction energies between cation and anion in ionic liquids: the subtle balance between coulomb forces and hydrogen bonding. J Mol Liq 192:94–102
Fujii K, Seki S, Ohara K, Kameda Y, Doi H, Saito S, Umebayashi Y (2014) High-energy X-ray diffraction and MD simulation study on the ion-ion interactions in 1-ethyl-3-ethylimidazolium bis (fluorosulfonyl) amide. J Solut Chem 43:1655–1668
Liu X, Li S, Wang D, Ma Y, Liu X, Ning M (2015) Theoretical study on the structure and cation–anion interaction of triethylammonium chloroaluminate ionic liquid. Computational and Theoretical Chemistry. 1073:67–74
Voroshylova IV, Teixeira F, Costa R, Pereira CM, Cordeiro MND (2016) Interactions in the ionic liquid [EMIM][FAP]: a coupled experimental and computational analysis. Phys Chem Chem Phys 18:2617–2628
Rathke DKB, Kiefer J (2016) Materny, A. Molecular structure and interactions in the ionic liquid 1-ethyl-3-methylimidazolium trifluoromethanesulfonate. J Phys Chem A 120:6274–6286
Mao JX, Damodaran K (2015) Spectroscopic and computational analysis of the molecular interactions in the ionic liquid [Emim]+[FAP]−. Ionics 21(6):1605–1613
Roohi H, Salehi R (2011) Molecular interactions in methylimidazolium tetrafluoroborate ionic liquid([Mim+][BF4 −]): Structures, binding energies, topological properties and NMR one- and two bonds spin–spin coupling constants. J Mol Liq 161:63–71
Roohi H, Khyrkhah S (2013) Ion-pairs formed in [Mim+][N(CN)2 −] ionic liquid: structures, binding energies, NMR SSCCs, volumetric, thermodynamic and topological properties. J Mol Liq 177:119–128
Roohi H, Khyrkhah S (2015) Quantum chemical studies on nanostructures of the hydrated methylimidazolium–based ionic liquids. J Mol Model 21:1–11
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital donor–acceptor viewpoint. Chem Rev 88:899–926
Zhao Y, Truhlar DG (2006) The M06 suite of density Functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new Functionals and systematic testing of four M06 functionals and twelve other functionals. Theor Chem Accounts 120:215–241
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J Chem Phys 72:5639–5648
Boys SF, de Bernardi F (1970) Calculation of small molecular interactions by differences of separate total energies−some procedures with reduced errors. J Mol Phys 19:553–566
Schwabe T, Grimme S (2006) Towards chemical accuracy for the thermodynamics of large molecules: New hybrid density functionals including non-local correlation effects. Phys Chem Chem Phys 8:4398–4401
Schwabe T, Grimme S (2007) Double-hybrid density functionals with long-range dispersion corrections: higher accuracy and extended applicability. Phys Chem Chem Phys 9:3397–3406
Grimme S, Antony J, Ehrlich S, Krieg HA (2010) Consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–154119
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry. VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GAA (1999) Complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822–2827
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery AJ, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox J, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision C.01. Gaussian Inc., Wallingford
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Biegler König FW, Schönbohm J, Bayles DJ (2001) Comput Chem 22:545, AIM2000.
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1998) NBO Version:3.1
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094 and references cited therein
Pascual-Ahuir JL, Silla E, Tunon I (1994) GEPOL: an improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J Comput Chem 15:1127–1138
Levine IN (2013) Quantum chemistry, 7th edn, Pearson.
Eckert F, Klamt A (2002) Fast solvent screening via quantum chemistry:COSMO-RS approach. AICHE J 48:369–385
Klamt A (1995) Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J Phys Chem 99:2224–2235
Dong K, Zhang S, Wang D, Yao X (2006) Hydrogen bonds in imidazolium ionic liquids. J Phys Chem A 110:9775–9782
Schulz T, Ahrens S, Meyer D, Allolio C, Peritz A, Strassner T (2011) Electronic effects of para-substitution on the melting points of TAAILs. Chem Asian J 6:863–867
Tsuzuki S, Tokuda H, Hayamizu K et al (2005) Magnitude and directionality of interaction in ion pairs of ionic liquids: relationship with ionic conductivity. J Phys Chem B 109:16474–16481
Roohi H, Ghauri K (2015) Exploring physicochemical properties of the nanostructured tunable Aryl Alkyl Ionic Liquids (TAAILs). J Mol Liq 209:14–24
Burns LA, Vázquez-Mayagoitia Á, Sumpter BG, Sherrill CD (2011) Density-functional approaches to noncovalent interactions: a comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functionals. J Chem Phys 134:084107–084125
Cybulski SM, Lytle ML (2007) The origin of deficiency of the supermolecule second-order Møller-Plesset approach for evaluating interaction energies. J Chem Phys 127:141102–141104
Lao KU, Schaffer R, Jansen G, Herbert JM (2015) Accurate description of intermolecular interactions involving ions using symmetry-adapted perturbation theory. J Chem Theory Comput 11:2473–2486
Khrizman A, Cheng HY, Bottini G, Moyna G (2015) Observation of aliphatic C–H⋯ X hydrogen bonds in imidazolium ionic liquids. Chem Commun 51:3193–3195
Katsyuba SA, Vener MV, Zvereva EE, Fei Z, Scopelliti R, Laurenczy G, Dyson PJ (2013) How strong is hydrogen bonding in ionic liquids? Combined X-ray crystallographic, infrared/Raman spectroscopic, and density functional theory study. J Phys Chem B 117:9094–9105
Roohi H, Ghauri K (2016) Influence of various anions and cations on electrochemical and physicochemical properties of the nanostructured Tunable Aryl Alkyl Ionic Liquids (TAAILs): A DFT M06-2X study. Thermochim Acta 639:20–40
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68:441–451
Jin X, Hu B, Jia H, Liu Z, Lu C (2014) Structure, thermal behaviour, and energetic properties of 4-amino-1,2,4-triazole dinitroguanidine salt. Aust J Chem 67:277–282
Drake G, Hawkins T, Brand A, Hall L, Mckay M, Vij A, Ismail I (2003) Energetic, low-melting salts of simple heterocycles. Propellants Explos Pyrotech 28:174–180
Emel'yanenko VN, Zaitsau DH, Verevkin SP, Heintz A (2011) Vaporization and formation enthalpies of 1-alkyl-3-methylimidazolium tricyanomethanides. J Phys Chem B 115:11712–11717
Verevkin SP, Emel'yanenko VN, Zaitsau DH, Heintz A, Muzny CD, Frenkelb M (2010) Thermochemistry of imidazolium-based ionic liquids: experiment and first-principles calculations. Phys Chem Chem Phys 12:14994–15000
Eckert F, Klamt A (2006) COSMOtherm Version C2.1 Release 01.08, COSMO logic GmbH & Co. KG, Leverkusen, Germany.
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem 24:669–681
Takano Y, Houk KN (2005) Benchmarking the conductor-like polarizable continuum model (CPCM) for aqueous solvation free energies of neutral and ionic organic molecules. J Chem Theory Comput 1:70–77
Palomar J, Ferro VR, Torrecilla JS, Rodrıguez F (2007) Density and molar volume predictions using COSMO-RS for ionic liquids. An approach to solvent design. Industrial and Engineering Chemistry Research 46:6041–6048
Hobza P, Havlas Z (2000) Blue-shifting hydrogen bonds. Chem Rev 100:4253–4264
Reed AE, Curtiss LA, Weinhold F (1998) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Hobza P, Spirko V, Selzle HL, Schlag EW (1998) Anti-hydrogen bond in the benzene dimer and other carbon proton donor complexes. J Phys Chem A 102:2501–2504
Koch U, Popelier PLA (1995) Characterization of CHO hydrogen bonds on the basis of the charge density. J Phys Chem 99:9747–9754
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102:873–1878
Endres F, El Abedin SZ (2006) Air and water stable ionic liquids in physical chemistry. Phys Chem Chem Phys 8:2101–2116
Wasserscheid P, Welton T (2008) Ionic liquids in synthesis. Wiley-VCH, Weinheim
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roohi, H., Salehi, R. Molecular engineering of the electronic, structural, and electrochemical properties of nanostructured 1-methyl-4-phenyl 1,2,4 triazolium-based [PhMTZ][X1–10] ionic liquids through anionic changing. Ionics 24, 483–504 (2018). https://doi.org/10.1007/s11581-017-2198-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2198-3