Abstract
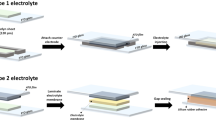
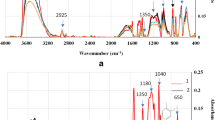
A poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS)-TiO2 composite layer was fabricated using a nanoparticle deposition system (NPDS) followed by spin coating process. The charge balance and transmittance change of electrochromic (EC) devices were investigated with different electrolytes, such as LiClO4-based polymer electrolyte and ionic liquid (IL). It was found that the EC properties could be improved using 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (EMIM-TFSI) with high ionic conductivity, and a symmetric charge balance change can be obtained showing rapid switching. The PEDOT:PSS-TiO2 composite exhibited stable performance without degradation after 500 cycles, maintaining the same cyclic voltammetry (CV) curve. It seems that heterostructure of TiO2 with PEDOT:PSS showed good separation of electrons and holes, making TiO2 an efficient electron transport material with PEDOT:PSS being an efficient hole transport material. Thus, it was found that the organic-inorganic structure obtained by our simple and novel process was effective in improving EC properties due to efficient separation of electrons and holes.











Similar content being viewed by others
References
Beaujuge PM, Reynolds JR (2010) Color control in π-conjugated organic polymers for use in electrochromic devices. Chem Rev 110(1):268–320
Lampert CM (2003) Large-area smart glass and integrated photovoltaics. Sol Energy Mater Sol Cells 76(4):489–499
Monk PM, Delage F, Vieira SMC (2001) Electrochromic paper: utility of electrochromes incorporated in paper. Electrochim Acta 46(13):2195–2202
Tehrani P, Hennerdal L-O, Dyer AL, Reynolds JR, Berggren M (2009) Improving the contrast of all-printed electrochromic polymer on paper displays. J Mater Chem 19(13):1799–1802
Gillaspie DT, Tenent RC, Dillon AC (2010) Metal-oxide films for electrochromic applications: present technology and future directions. J Mater Chem 20(43):9585–9592
Thakur VK, Ding G, Ma J, Lee PS, Lu X (2012) Hybrid materials and polymer electrolytes for electrochromic device applications. Adv Mater 24(30):4071–4096
Deb S (1969) A novel electrophotographic system. Appl Opt 8(101):192–195
Cummins D, Boschloo G, Ryan M, Corr D, Rao SN, Fitzmaurice D (2000) Ultrafast electrochromic windows based on redox-chromophore modified nanostructured semiconducting and conducting films. J Phys Chem B 104(48):11449–11459
Lu W, Fadeev AG, Qi B, Mattes BR (2004) Fabricating conducting polymer electrochromic devices using ionic liquids. J Electrochem Soc 151(2):H33–H39
Thompson BC, Schottland P, Zong K, Reynolds JR (2000) In situ colorimetric analysis of electrochromic polymers and devices. Chem Mater 12(6):1563–1571
Xu C, Liu L, Legenski SE, Ning D, Taya M (2004) Switchable window based on electrochromic polymers. J Mater Res 19(07):2072–2080
Corradini A, Marinangeli A, Mastragostino M (1990) Ito as counter-electrode in a polymer based electrochromic device. Electrochim Acta 35(11–12):1757–1760
Ling H, Lu J, Phua S, Liu H, Liu L, Huang Y, Mandler D, Lee PS, Lu X (2014) One-pot sequential electrochemical deposition of multilayer poly (3, 4-ethylenedioxythiophene): poly (4-styrenesulfonic acid)/tungsten trioxide hybrid films and their enhanced electrochromic properties. J Mater Chem A 2(8):2708–2717
Zhu J, Wei S, Zhang L, Mao Y, Ryu J, Karki AB, Young DP, Guo Z (2011) Polyaniline-tungsten oxide metacomposites with tunable electronic properties. J Mater Chem 21(2):342–348
Liu S, Xu L, Li F, Guo W, Xing Y, Sun Z (2011) Carbon nanotubes-assisted polyoxometalate nanocomposite film with enhanced electrochromic performance. Electrochim Acta 56(24):8156–8162
Sakai N, Prasad GK, Ebina Y, Takada K, Sasaki T (2006) Layer-by-layer assembled TiO2 nanoparticle/PEDOT-PSS composite films for switching of electric conductivity in response to ultraviolet and visible light. Chem Mater 18(16):3596–3598
Ma L, Li Y, Yu X, Yang Q, Noh C-H (2008) Using room temperature ionic liquid to fabricate PEDOT/TiO2 nanocomposite electrode-based electrochromic devices with enhanced long-term stability. Sol Energy Mater Sol Cells 92(10):1253–1259
Huang S-W, Ho K-C (2006) An all-thiophene electrochromic device fabricated with poly (3-methylthiophene) and poly (3, 4-ethylenedioxythiophene). Sol Energy Mater Sol Cells 90(4):491–505
Smela E (1998) Thiol-modified pyrrole monomers: 4. Electrochemical deposition of polypyrrole over 1-(2-thioethyl) pyrrole. Langmuir 14(11):2996–3002
Sayre CN, Collard DM (1995) Self-assembled monolayers of pyrrole-containing alkanethiols on gold. Langmuir 11(1):302–306
Chun D-M, Ahn S-H (2011) Deposition mechanism of dry sprayed ceramic particles at room temperature using a nano-particle deposition system. Acta Mater 59(7):2693–2703
Chun D-M, Choi J-O, Lee CS, Kanno I, Kotera H, Ahn S-H (2012) Nano-particle deposition system (NPDS): low energy solvent-free dry spray process for direct patterning of metals and ceramics at room temperature. Int J Precis Eng Manuf 13(7):1107–1112
Kyubong J, Woojin S, Doo-Man C, Yang-Hee K, Jun-Cheol Y, Min-Saeng K, Sung-Hoon A, Caroline Sunyong L (2010) Nickel line patterning using silicon supersonic micronozzle integrated with a nanoparticle deposition system. Jpn J Appl Phys 49(5S1):05EC09
Song W, Jung K, D-M C, S-H A, Lee CS (2010) Deposition of Al2O3 powders using nano-particle deposition system. Surf Rev Lett 17(02):189–193. doi:10.1142/S0218625X10013710
Kim K-S, Lee J, Kim YH, Lee CS (2013) Effect of scanning speed on copper line deposition using nanoparticle deposition system (NPDS) for direct printing technology. Aerosol Sci Technol 47(1):106–113
Kim H, Yang S, Ahn S-H, Lee CS (2016) Effect of particle size on various substrates for deposition of NiO film via nanoparticle deposition system. Thin Solid Films 600:109–118
Kim H, Yang S, Pawar RC, Ahn S-H, Lee CS (2015) Role of TiO2 nanoparticles in the dry deposition of NiO micro-sized particles at room temperature. Ceram Int 41(4):5937–5944
Yang S, Kim H, Ahn S-H, Lee CS (2015) The effect of the agglomerated microstructure of dry-deposited TiO2 electrodes on the performance of dye-sensitized solar cells. Electrochim Acta 166:117–123
Ahn S-H (2014) An evaluation of green manufacturing technologies based on research databases. Int J Precis Eng Manuf Green Technol 1(1):5–9
Park S-I, Kim S, Choi J-O, Song J-H, Taya M, Ahn S-H (2015) Low-cost fabrication of WO 3 films using a room temperature and low-vacuum air-spray based deposition system for inorganic electrochromic device applications. Thin Solid Films 589:412–418
Kim H, Park Y, Choi D, Ahn S-H, Lee CS (2016) Novel fabrication of an electrochromic antimony-doped tin oxide film using a nanoparticle deposition system. Appl Surf Sci 377:370–375
Lu W, Fadeev AG, Qi B, Smela E, Mattes BR, Ding J, Spinks GM, Mazurkiewicz J, Zhou D, Wallace GG (2002) Use of ionic liquids for π-conjugated polymer electrochemical devices. Science 297(5583):983–987
Zhang AJ, Qi XM, Du YF, Luo YW, Fang HJ, Lu JX (2006) Electropolymerization of 3-methylthiophene in [BMIM] PF6 ionic liquid, characterization and application. Chin J Chem 24(5):609–612
Ribeiro A, Machado D, dos Santos Filho PF, De Paoli M-A (2004) Solid-state electrochromic device based on two poly (thiophene) derivatives. J Electroanal Chem 567(2):243–248
Won J, Kim D, Kim H (2004) Ionic liquids for electrolytes. J Polym Sci Technol 15(4):449–456
Sekiguchi K, Atobe M, Fuchigami T (2002) Electropolymerization of pyrrole in 1-ethyl-3-methylimidazolium trifluoromethanesulfonate room temperature ionic liquid. Electrochem Commun 4(11):881–885
Sekiguchi K, Atobe M, Fuchigami T (2003) Electrooxidative polymerization of aromatic compounds in 1-ethyl-3-methylimidazolium trifluoromethanesulfonate room-temperature ionic liquid. J Electroanal Chem 557:1–7
Rogers RD, Seddon KR (2003) Ionic liquids—solvents of the future? Science 302(5646):792–793
Park S, Yi C, Kim H, Cho W, Cho B, Yun K, An C, Woo K (1995) Electrical and optical properties of electrochromic window with both lithium and proton conducting polymer electrolytic media. J Korean Inst Surf Eng 28(1):46–54
Stenman A (2013) Electrochromic properties of nickel oxide in different electrolytes. Dissertation, Uppsala University
Bohnke C, Bohnke O (1990) Impedance analysis of amorphous WO3 thin films in hydrated LiClO4-propylene carbonate electrolytes. Solid State Ionics 39(3–4):195–204
Qian X, Gu N, Cheng Z, Yang X, Wang E, Dong S (2001) Methods to study the ionic conductivity of polymeric electrolytes using ac impedance spectroscopy. J Solid State Electrochem 6(1):8–15
Takagi S, Makuta S, Veamatahau A, Otsuka Y, Tachibana Y (2012) Organic/inorganic hybrid electrochromic devices based on photoelectrochemically formed polypyrrole/TiO2 nanohybrid films. J Mater Chem 22(41):22181–22189
Hepel M, Redmond H (2009) Large cation model of dissociative reduction of electrochromic WO3− x films. Open Chem 7(2):234–245
Hepel M, Redmond H, Dela I (2007) Electrochromic WO 3− x films with reduced lattice deformation stress and fast response time. Electrochim Acta 52(11):3541–3549
Shiyanovskaya I, Hepel M, Tewksburry E (2000) Electrochromism in electrodeposited nanocrystalline WO~ 3 films I. Electrochemical and optical properties. J New Mater Electrochem Syst 3(3):241–248
Nah Y-C, San Choi W, Kim D-Y (2008) Preparation and electrochromic properties of spin self-assembled polyelectrolyte multilayer films composed of PEDOT: PSS and PAH. Sol Energy Mater Sol Cells 92(12):1547–1551
Ling H, Liu L, Lee PS, Mandler D, Lu X (2015) Layer-by-layer assembly of PEDOT: PSS and WO 3 nanoparticles: enhanced electrochromic coloration efficiency and mechanism studies by scanning electrochemical microscopy. Electrochim Acta 174:57–65
Liao J, Ho K (2005) A photoelectrochromic device using a PEDOT thin film. J New Mater Electrochem Syst 8:37–47
Lu J, Song H, Li S, Wang L, Han L, Ling H, Lu X (2015) A poly (3, 4-ethylenedioxythiophene): poly (styrene sulfonic acid)/titanium oxide nanocomposite film synthesized by sol–gel assisted electropolymerization for electrochromic application. Thin Solid Films 584:353–358
Jin ZC, Hamberg I, Granqvist C (1988) Optical properties of sputter-deposited ZnO: Al thin films. J Appl Phys 64(10):5117–5131
Acknowledgments
This work was supported by the Energy Efficiency & Resources Core Technology and Human Resources Development Program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resources from the Ministry of Trade, Industry & Energy, Republic of Korea (Grant No. 20142020103730 and No. 20154030200680). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of education) (No.NPF-2016R1D1A1A02936936).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 150 kb)
Rights and permissions
About this article
Cite this article
Kim, H., Kim, K., Choi, D. et al. Evaluation of a reliable electrochromic device based on PEDOT:PSS-TiO2 heterostructure fabricated at low temperature. Ionics 23, 2465–2474 (2017). https://doi.org/10.1007/s11581-017-2192-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2192-9