Abstract
Doped ceria-carbonate electrolytes have high ionic conductivity and shows good solid oxide fuel cell (SOFC) performance at low temperature (400–600 °C). Various compositions of unary, binary, and ternary gadolinium-doped ceria-carbonate electrolytes are prepared using (Na, Li, K, Sr)-CO3 and GDC. The electrolytes are physically and electrochemically characterized using X-ray diffraction, scanning electron microscopy, energy dispersive spectroscopy, and two-probe AC-conductivity methods. In the unary gadolinium-doped ceria-carbonate composite electrolytes, best ionic conductivity is shown by 25 wt% Li2CO3-GDC electrolyte (0.077 S cm−1 at 600 °C). The ternary carbonate composite mixture of 25 wt% (LiNaK)2CO3-GDC exhibits higher ionic conductivity (0.29 S cm−1 at 600 °C) than binary and unary carbonate electrolytes. The enhanced conductivity may be due to lower eutectic temperature of (LiNaK)2CO3 rendering larger (LiNaK)2CO3-GDC interface. At 600 °C, the peak power density for the cell of ternary carbonate, 25 wt% (LiNaK)2CO3-GDC electrolyte and binary carbonate, 25 wt% (LiNa)2CO3-GDC electrolyte is 224 mW cm−2 (at 555 mA cm−1) and 180 mW cm−2 (at 417 mA cm−1), which would enable them to be used as electrolytes for low-temperature SOFC.

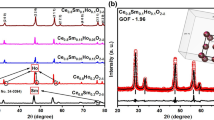
Current-voltage characteristics and SEM micrograph of 25 wt% (LiNaK)2CO3-GDC electrolyte and 25 wt% (LiNa)2CO3-GDC electrolyte.





Similar content being viewed by others
References
Singhal SC, Kendall K (2003) High temperature solid oxide fuel cells: fundamentals, design, applications. Elsevier Science Ltd., Amsterdam
Steele BCH (2000) Appraisal of Ce1-yGdyO2-y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ionics 129:95–110
Brandon NP, Skinner S, Steele BCH (2003) Recent advances in materials for fuel cells. Annu Rev Mater Res 33:183–213
Steele BCH (1999) Fuel-cell technology: running on natural gas. Nature 400:619–621
Haile SM (2003) Fuel cell materials and components. Acta Mater 51:5981–6000
Milliken C, Guruswamy S, Khandkar A (1999) Evaluation of ceria electrolytes in solid oxide fuel cells electric power generation. J Electrochem Soc 146:872–882
Xia C, Liu M (2001) Low-temperature SOFCs based on Gd0.1Ce0.9O1.95 fabricated by dry pressing. Solid State Ionics 144:249–255
Steele BCH, Heinzel A (2001) A materials for fuel-cell technologies. Nature 414:345–352
Liang C (1973) Conduction characteristics of the lithium iodide-aluminium oxide solid electrolytes. J Electrochem Soc 120:1289–1292
Huang JB, Yang LZ, Gao RF, Mao ZQ, Wang C (2006) A high-performance ceramic fuel cell with samarium doped ceria–carbonate composite electrolyte at low temperatures. Electrochem Commun 8:785–789
Zhu B, Yang XT, Xu J, Zhu ZG, Ji SJ, Sun MT, Sun JC (2003) Innovative low temperature SOFCs and advanced materials. J Power Sources 118:47–53
Schober T (2005) Composites of ceramic high-temperature proton conductors with inorganic compounds. Electrochem Solid-State Lett 8:A199–A200
Demin A, Tsiakaras P, Gorbova E, Hramova S (2004) A SOFC based on a co-ionic electrolyte. J Power Sources 131:231–236
Li Y, Rui Z, Xia C, Anderson M, Lin YS (2009) Performance of ionic-conducting ceramic/carbonate composite material as solid oxide fuel cell electrolyte and CO2 permeation membrane. Catal Today 148:303–309
Lapa CM, Figueiredo FML, De Souza DPF, Song L, Zhu B, Marques FMB (2010) Synthesis and characterization of composite electrolytes based on samaria-doped ceria and Na/Li carbonates. Int J Hydrog Energy 35:2953–2957
Zhu B (2003) Functional ceria–salt-composite materials for advanced ITSOFC applications. J Power Sources 114:1–9
Raza R, Qin H, Fan L, Takeda K, Mizuhata M, Zhu B (2012) Electrochemical study on co-doped ceria-carbonate composite electrolyte. J Power Sources 9201:121–127
Xia C, Li Y, Tian Y, Liu Q, Zhao Y, Jia L, Li Y (2009) A high performance composite ionic conducting electrolyte for intermediate temperature fuel cell and evidence for ternary ionic conduction. J Power Sources 188:156–162
Huang J, Gao Z, Mao Z (2010) Effects of salt composition on the electrical properties of samaria-doped ceria/carbonate composite electrolytes for low-temperature SOFCs. Int J Hydrog Energy 35:4270–4275
Jing Y, Patakangas J, Lund PD, Zhu B (2013) An improved synthesis method of ceria-carbonate based composite electrolytes for low-temperature SOFC fuel cells. Int J Hydrog Energy 38:16532–16538
Xia C, Li Y, Tian Y, Liu Q, Wang Z, Zhao Y, Jia L, Li Y (2010) Intermediate temperature fuel cell with a doped ceria–carbonate composite electrolyte. J Power Sources 195:3149–3154
Chockalingam R, Jain S, Basu S (2010) Studies on conductivity of composite GdCeO2-carbonate electrolytes for low temperature solid oxide fuel cells. Integr Ferroelectr 116:23–34
Huang JB, Mao ZQ, Yang LZ, Peng RR (2005) SDC–carbonate composite electrolytes for low-temperature SOFCs. Electrochem Solid-State Lett 8(9):A437–A440
Näfe H (2014) Conductivity of alkali carbonates, carbonate-based composite electrolytes and IT-SOFC. ECS J Solid State Sci Technol 3:N7–N14
Selman JR, Maruin G, Mamantov HC, Braunstein J (1981) Advances in molten salt chemistry, vol 4. Plenum Press, New York, pp 159–389
Huang J, Mao Z, Liu Z, Wang C (2007) Development of novel low-temperature SOFCs with co-ionic conducting SDC-carbonate composite electrolytes. Electrochem Commun 9:2601–2605
Chockalingam R, Basu S (2011) Impedance spectroscopy studies of Gd-CeO2-(LiNa)CO3 nanocomposite electrolyte for low temperature SOFC applications. Int J Hydrog Energy 386:14977–14983
Raza R, Wang X, Mac Y, Liu X, Zhu B (2010) Improved ceria-carbonate composite electrolytes. Int J Hydrog Energy 35:2684–2688
Wang X, Ma Y, Li S, Zhu B, Muhammed M (2012) SDC/Na2CO3 nanocomposite: new freeze drying based synthesis and application as electrolyte in low-temperature solid oxide fuel cells. Int J Hydrog Energy 37:19380–19387
Fan L, Zhang G, Chen M, Wang C, Di J, Zhu B (2012) Proton and oxygen ionic conductivity of doped ceria-carbonate composite by modified Wagner polarization. Int J Electrochem Sci 7:8420–8435
Fan L, Wang C, Chen M, Zhu B (2013) Recent developments of ceria-based (nano)composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells. J Power Sources 234:154–174
Khan MA, Raza R, Lima RB, Chaudhry MA, Ahmed E, Abbas G (2013) Comparative study of the nano-composite electrolytes based on samaria-doped ceria for low temperature solid oxide fuel cells (LT-SOFCs). Int J Hydrog Energy 38:16524–16531
Li C, Zeng Y, Wang Z, Xu F, Ye Z, Shi R (2016) An investigation of protonic and oxide ionic conductivities at the interfacial layers in SDC-LNC composite electrolytes. Electrochim Acta 212:583–593
Yin S, Zeng Y, Li C, Chen X, Ye Z (2013) Investigation of Sm0.2Ce0.8O1.9/Na2CO3 nanocomposite electrolytes: preparation, interfacial microstructures, and ionic conductivities. ACS Appl Mater Interfaces 5:12876–12886
Janz GJ, Lorenz MR (1961) Molten carbonate electrolytes: physical properties, structure and mechanism of electrical conductance. J Electrochem Soc 108:1052–1058
Spedding PL (1973) Electrical conductance of molten alkali carbonate binary mixtures. J Electrochem Soc 120:1049–1052
Miyazaki Y, Yanagida M, Tanimoto K, Kodama T, Tanase S (1986) An apparatus for electrical conductance measurements with molten carbonates. J Electrochem Soc 133:1402–1404
Kojima T, Miyazaki Y, Nomura K, Tanimoto K (2007) Electrical conductivity of molten Li2CO3-X2CO3 (X: Na, K, Rb, and Cs) and Na2CO3-Z2CO3 (Z: K, Rb, and Cs). J Electrochem Soc 154:F222–F230
Cerisier P, Roux F (1977) A study of the electrical conductivity and transition points of sodium carbonate. J Solid State Chem 22:245–251
Cerisier P, Roux F (1978) A study of the electrical conductivity and transition points of potassium carbonate. Solid State Commun 26:661–663
Ward AT, Janz GJ (1965) Molten carbonate electrolytes: electrical conductance, density and surface tension of binary and ternary mixtures. Electrochim Acta 10:849–857
Kojima T, Miyazaki Y, Nomura K, Tanimoto K (2008) Density, surface tension, and electrical conductivity of ternary molten carbonate system Li2CO3 – Na2CO3 – K2CO3 and methods for their estimation. J Electrochem Soc 155:F150–F156
Tanase S, Miyazaki Y, Yanagida M, Tanimoto K, Kodama T (1988) Work on electrically conductive materials for molten carbonate fuel cells. Prog Batter Solar Cells 7:389–395
Ferreira ASV, Soares CMC, Figueiredo FMHLR, Marques FMB (2011) Intrinsic and extrinsic compositional effects in ceria/carbonate composite electrolyte for fuel cells. Int J Hydrog Energy 36:3704–3711
Ali SAM, Muchtar A, Sulong AB, Muhamad N, Majlan EH (2013) Influence of sintering temperature on the power density of samarium-doped-ceria carbonate electrolyte composites for low-temperature solid oxide fuel cells. Ceram Int 39:5813–5820
Fan L, He C, Zhu B (2017) Role of carbonate phase in ceria–carbonate composite for low temperature solid oxide fuel cells: a review. Int J Energy Res 41:465–481
Acknowledgements
Authors would like to acknowledge funding received for executing the project from Inno-Indigo project scheme between the European Union and DST, Government of India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 425 kb)
Rights and permissions
About this article
Cite this article
Khan, I., Tiwari, P.K. & Basu, S. Analysis of gadolinium-doped ceria-ternary carbonate composite electrolytes for solid oxide fuel cells. Ionics 24, 211–219 (2018). https://doi.org/10.1007/s11581-017-2184-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2184-9